Varadia amboliensis Bhosale, Thackeray, Muley & Raheem, 2021
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.757.1413 |
|
publication LSID |
lsid:zoobank.org:pub:7EC5E08C-7E6C-4C06-A0C6-1346A4A30A10 |
|
DOI |
https://doi.org/10.5281/zenodo.5056010 |
|
persistent identifier |
https://treatment.plazi.org/id/5C93F719-2DEF-4A9A-8973-06B7FE6E6528 |
|
taxon LSID |
lsid:zoobank.org:act:5C93F719-2DEF-4A9A-8973-06B7FE6E6528 |
|
treatment provided by |
Felipe |
|
scientific name |
Varadia amboliensis Bhosale, Thackeray, Muley & Raheem |
| status |
gen. et sp. nov. |
Varadia amboliensis Bhosale, Thackeray, Muley & Raheem gen. et sp. nov.
urn:lsid:zoobank.org:act:5C93F719-2DEF-4A9A-8973-06B7FE6E6528
Figs 3–12 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Diagnosis
As genus-level diagnosis.
Etymology
Named after the type locality, Amboli, in the Sindhudurg District of southern Maharashtra, India. In recent years, Amboli has emerged as a hotspot for the discovery of new species (particularly reptiles and amphibians) in the northern Western Ghats.
Type material
Holotype
INDIA • Maharashtra State, Sindhudurg District, Amboli, Hiranyakeshi temple; 15°57′17.8″ N, 74°01′39.1″ E; 839 m a.s.l.; 2019; A. Bhosale leg.; BNHS GAS 113 About GAS . GoogleMaps
Paratypes
INDIA • 21 specimens (17 whole preserved specimens and 4 shells); same locality data as for holotype; 2019; A. Bhosale leg.; BNHS GAS 114–127 About GAS , ZSI Moll/1820–1826 • 3 preserved specimens; same locality data as for holotype; 2020; A. Bhosale leg.; BNHS GAS 136–138 About GAS .
Other material examined
INDIA – Maharashtra State • 1 specimen (sampled for DNA analysis); Sindhudurg District, Amboli Forest Park; 15°57′37.4″ N, 73°59′58.1″ E; 724 m a.s.l.; 2017; A. Bhosale leg.; BNHS GAS 129 About GAS GoogleMaps • 9 preserved specimens; Sindhudurg District, near Amboli waterfall; 15°56′26.9″ N, 73°59′41.2″ E; 645 m a.s.l.; 2020; A. Bhosale leg.; BNHS GAS 130–135 About GAS , BNHS GAS 139–141 About GAS GoogleMaps • 1 shell; Kolhapur District, Kodali ; 15°46′42.4″ N, 74°10′40.0″ E; 620 m a.s.l.; 2019; A. Bhosale leg.; BNHS GAS 128 About GAS GoogleMaps .
Description
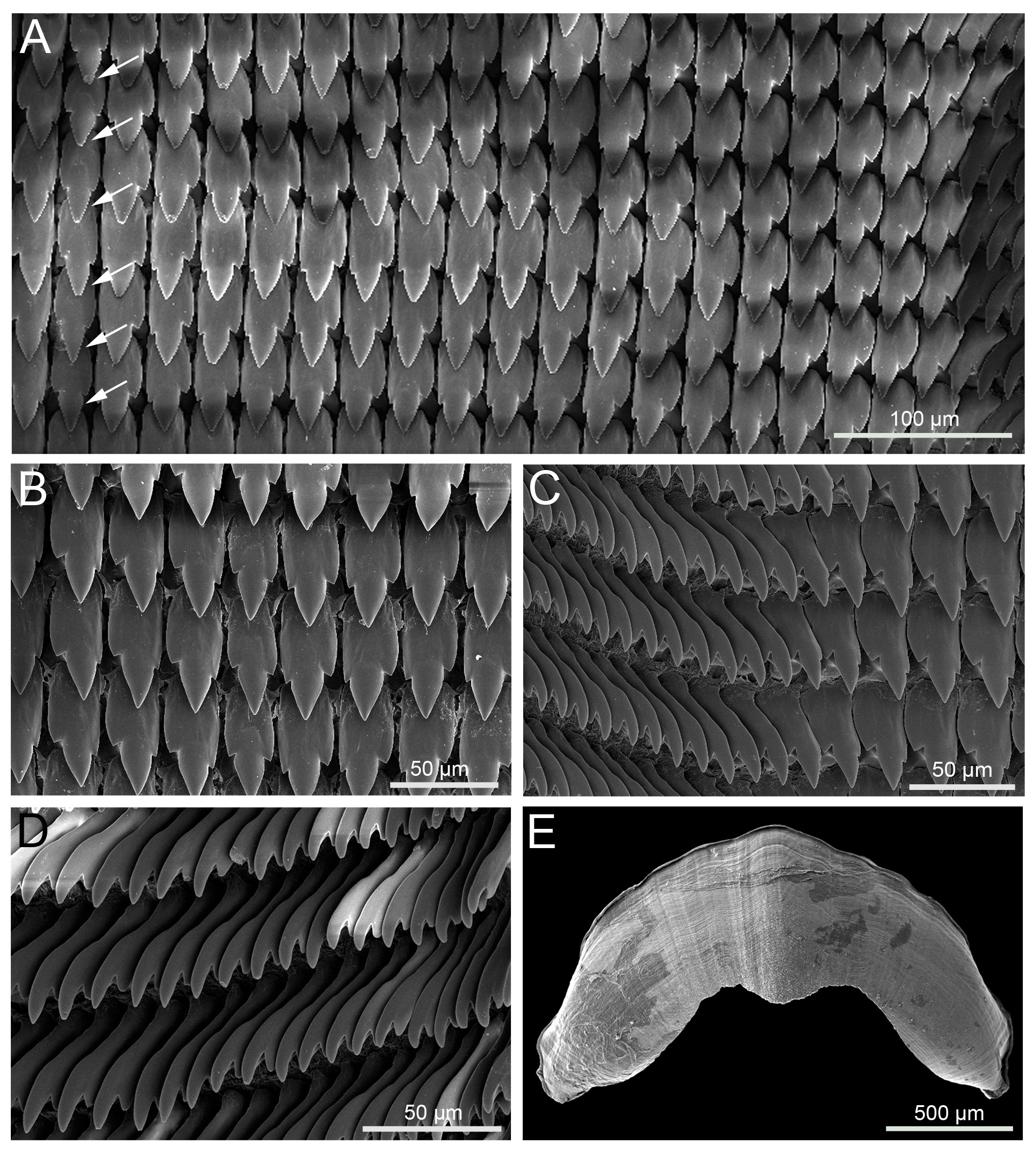
SHELL.Adult shell thin, depressed, glossy and appearing non-umbilicate, with ca 4–4.5 rapidly increasing whorls and colour ranging from golden-brown to reddish yellow ( Fig. 3 View Fig ). Shell measurements (n = 35): width 20.2–26.3 mm; height 10.2–15.0 mm. Spire only slightly raised with flat apex and suture only slightly impressed. Body whorl disproportionately large, rounded at periphery, gently convex beneath. Aperture large, crescent-shaped, with width greater than height ( Fig. 3A, E View Fig ). Apertural margin simple, thin and delicate; in lateral view angled forward, with upper apertural margin noticeably anterior to the lower margin. When shell is viewed from below, basal margin curved (not straight) and expanded columellar margin reflected over, covering umbilical region. Shell surface smooth and glossy to naked eye, with irregular, faint collabral striae; under SEM, seen to be finely and closely sculptured with well-defined spiral lines on protoconch (first 1.5–2 whorls) and indistinct and irregular oblique lines on teleoconch ( Fig. 4 View Fig ).
BODY AND MANTLE. Total adult body length, excluding extended tentacles, ranges from 4.8 to 6.9 cm (n = 5). Living snail glossy grey or greyish white with irregular dark mottling; head and tail dark grey or blackish with tentacles tending to be paler at their tips ( Fig. 5–6 View Fig View Fig ). Surface of mantle densely and conspicuously covered by small, irregular tubercles that appear lighter on top. Sole tripartite with welldefined sole furrows dividing it into three distinct longitudinal tracts; central tract paler than lateral ones. Tail with large slit-like caudal pit (sensu Hausdorf 1998: 51); caudal horn prominent when extended ( Fig. 6A View Fig ) but when retracted gives tail truncated and blunt appearance ( Fig. 6B View Fig ). Mantle consists of two broad shell lobes (right and left) and two dorsal lobes (right and left) ( Fig. 7 View Fig ). Shell lobes may cover nearly all of dorsal surface of shell ( Fig. 5 View Fig ), but individuals have also been observed with shell lobes largely retracted and much of shell exposed ( Fig. 6 View Fig ). Left dorsal lobe extends as far as base of tentacles when snail is resting (i.e., body not fully extended) and tentacles are retracted.
REPRODUCTIVE SYSTEM. Male genitalia consist of proximally penis and distally epiphallic caecum (= epiphallic retractor caecum of Hyman & Ponder 2010: 139) and epiphallus. These three regions are
held together by a penial sheath (= penial tunica of Hausdorf 1998), which is largely independent of wall of penis, epiphallic caecum and epiphallus ( Figs 8A View Fig , 9A–C View Fig ). Penial sheath encloses all of penis, all of epiphallic caecum, much of epiphallus and part of flagellum; it holds proximal three quarters of epiphallus in a loop against epiphallic caecum, with remaining part of epiphallus (i.e., part closest to vas deferens and flagellum) lying outside sheath, along with a substantial part of flagellum ( Fig. 9A View Fig ). Proximal part of penial sheath is thick and covers penis; distal half of this sheath is thin and covers epiphallus and epiphallic caecum. Thick penial sheath attached to proximal end of penis, close to genital atrium. Thin penial sheath attached to distal end of epiphallic caecum and is open where penial retractor muscle inserts on epiphallic caecum ( Fig. 9A–C View Fig ); an extension of the thin penial sheath also encloses a sizeable section of flagellum (this section located about halfway along length of flagellum). Epiphallus passes through and is attached to penial sheath in region where thick penial sheath transitions into thin penial sheath.
With penial sheath dissected open, penis seen to have noticeable S-shaped bend midway; this bend is associated with a band of muscle that extends for some distance along penis, on either side of bend ( Fig. 9D–E View Fig ). Distally, penis branches into wider-lumened epiphallic caecum and narrower-lumened epiphallus. Epiphallus passes into much narrower-lumened vas deferens; junction between these two regions marked by long, bluntly pointed flagellum, which is similar in length to epiphallus. Penial retractor muscle, which originates on inner lung wall, inserts in two places ( Fig. 9E View Fig ): subterminally on epiphallic caecum, and on apex of loop of epiphallus (i.e., about three quarters of distance from vas deferens to penis). Junction between two branches of penial retractor muscle located near most distal part of epiphallic caecum. Irregular small holes/pores visible on inner surface of thin part of penial sheath (i.e., with sheath cut open and pinned out).
On the basis of the morphology of its inner wall, penis divisible into three morphologically distinct regions, proximal penis, mid-penis and distal penis, with S-shaped bend of penis including all of mid- and
distal penial regions ( Fig. 10 View Fig ). Proximal penis shows one major and several minor longitudinal pilasters; close study at low magnification (4 ×) of holotype and one paratype (BNHS GAS 114) showed that pilasters are interspersed by fine, obliquely longitudinal ridges that are close and irregular. Mid-penis ornamented by several thin longitudinal pilasters. Distal penis also with thin longitudinal pilasters, but here they are fewer in number and are contiguous with uniform, widely spaced transverse ridges that extend outwards on either side of each pilaster. Opening of epiphallus into most proximal part of epiphallic caecum clearly visible ( Fig. 10 View Fig ). Inner wall of epiphallic caecum ( Fig. 10 View Fig ) has one major longitudinal pilaster (surface marked by irregular, fine longitudinal and/or transverse ridges; not shown in Fig. 10 View Fig ) running along its length; a large, reticulate mass of ridges proximally; and several short longitudinal pilasters distally. The short pilasters tend to be crenulated proximally and are smoother distally. Lumen of vas deferens widens with increasing distance from epiphallus, with part of vas deferens nearest to epiphallus being noticeably narrower-lumened than remaining two thirds ( Fig. 8A View Fig ). Right eye retractor muscle passes between male and female genitalia. Amatorial organ absent. Genital atrium cylindrical, well defined but short, with junction between male genitalia and vagina located at a short distance from genital orifice. Vagina cylindrical and shorter in length than genital atrium ( Fig. 8A View Fig ). Proximal part of oviduct, near junction with gametolytic gland, consists of pale yellowish, indistinctly-defined region, which is most likely the capsular gland (see Dasen 1933); inner wall of this gland irregularly marked by papillate ridges and papillae ( Fig. 8 View Fig ). Gametolytic gland ( Fig. 8A View Fig ) comprises narrow gametolytic duct and long, voluminous sac that is ca 3–3.5 times length of duct; duct noticeably constricted at its junction with sac and has 1–3 longitudinal ridges on its inner wall.
One or two spermatophores (i.e., only one wholly intact; the rest damaged/ partially digested) present in gametolytic gland of each of six specimens ( Fig. 11A View Fig ). Intact spermatophore consists of elongated, soft capsule with long tail-pipe. Sharply-angled, U-shaped bend at junction of capsule and tail-pipe; apex of bend noticeably hooked towards tail-pipe. Capsule wider-lumened than tail-pipe and twisted spirally ( Fig. 11B View Fig ). Tail-pipe flexible, internally hollow and externally sculptured obliquely along its length with four fine ribs. Tail-pipe in vicinity of tip hollow centrally and this passes into funnel-like opening (perforation) ( Fig. 11D View Fig ); surface of spermatophore near tip of tail-pipe has short, hair-like spines that point towards capsule ( Fig. 11C View Fig ).
RADULA AND JAW. Central tooth tricuspid, with large mesocone, which is shorter than tooth base, and smaller, more basal ectocones ( Fig. 12A–B View Fig ). Inner laterals 17–21, uniformly tricuspid ( Fig. 12A–B View Fig ); mesocone large, equal in size to those of central tooth and shorter than tooth base, endocone barely defined and ectocone prominent but more basal than other cusps. Outer 2 lateral teeth grade into marginal teeth. Marginal teeth 45–53, uniformly bicuspid (endocone absent), with shorter, narrower and more basal ectocone ( Fig. 12C–D View Fig ). Formulae for the 8 specimens examined are as follows (the plus sign indicates that the outermost marginal teeth could not be counted):
Holotype BNHS GAS 113 About GAS (+50.20.1.18.2.50+)
Paratype BNHS GAS 114 (+49.21.1.19.2.49+)
Paratype BNHS GAS 115 (53.19.1.17.2.53)
Paratype BNHS GAS 116 (+45.21.1.19.2.45+)
Paratype BNHS GAS 117 (+50.19.1.17.2.50+)
Paratype BNHS GAS 118 (+48.23.1.21.2.48+)
Paratype BNHS GAS 119 (+48.19.1.17.2.48+)
Paratype BNHS GAS 120 (52.22.1.20.2.52)
Jaw oxygnath (smooth), having a concave cutting edge with well-defined or barely evident median projection ( Fig. 12E View Fig ).
Distribution and ecology
Varadia amboliensis gen. et sp. nov. is endemic to the northern and central Western Ghats of India and is currently known from only 5 localities. These are: Hiranyakeshi temple, Amboli, Sindhudurg District, Maharashtra State (15°57′17.8″ N, 74°01′39.1″ E; 839 m a.s.l.); Amboli Forest Park, Sindhudurg District, Maharashtra State (15°57′37.4″ N, 73°59′58.1″ E; 724 m a.s.l.); near Amboli waterfall, Sindhudurg District, Maharashtra State (15°56′26.9″ N, 73°59′41.2″ E; 645 m a.s.l.); Kodali, Kolhapur District, Maharashtra State (15°46′42.4″N, 74°10′40.0″E; 620 m a.s.l.); Yana Forest, Uttara Kannada District, Karnataka State (14°35′16.4″N 74°34′00.3″E; 272 m a.s.l.) (A. Bhosale, 2018, personal observation). The species occurs at elevations ranging from 272 to 839 m. Although it has been observed among human habitation on forest edges ( Fig. 13 View Fig ), V. amboliensis gen. et sp. nov. appears to be primarily a species of tropical semi-evergreen and evergreen forest (sensu vegetation classification of Pascal 1991). The range of this species, as currently known, is restricted and disjunct. While 4 of the 5 known localities are in the extreme south of Maharashtra State (northern Western Ghats), the only other known locality, Yana Forest in northern Karnataka (central Western Ghats), is ca 160 km to the south. Further surveys are required to establish if this species occurs in the intervening area.
Varadia amboliensis gen. et sp. nov. is primarily a ground-living snail. It can be encountered at night in leaf litter or on rocks and the bases of trees; in rainy weather it can be seen on the exterior walls of buildings close to the forest edge (e.g., it was observed at the entrance of Amboli Forest Park in September 2017). The species can be seen throughout the monsoon (June to October) and as late as the end of November.
A few individuals have been seen in late February (late winter) on the banks of fast-flowing streams at Amboli.
This species appears to be omnivorous. It has been observed feeding on decaying plant matter (leaf litter, discarded banana peel) and on the remains of at least two different invertebrate taxa (a cricket and an earthworm) (A. Bhosale, personal observation) (Supp. file 1). Data on its predators are scarce,
but a scorpion of the genus Heterometrus Ehrenberg, 1828 (Scorpionidae) was observed feeding on an individual of this species (Supp. file 1).
DNA analysis
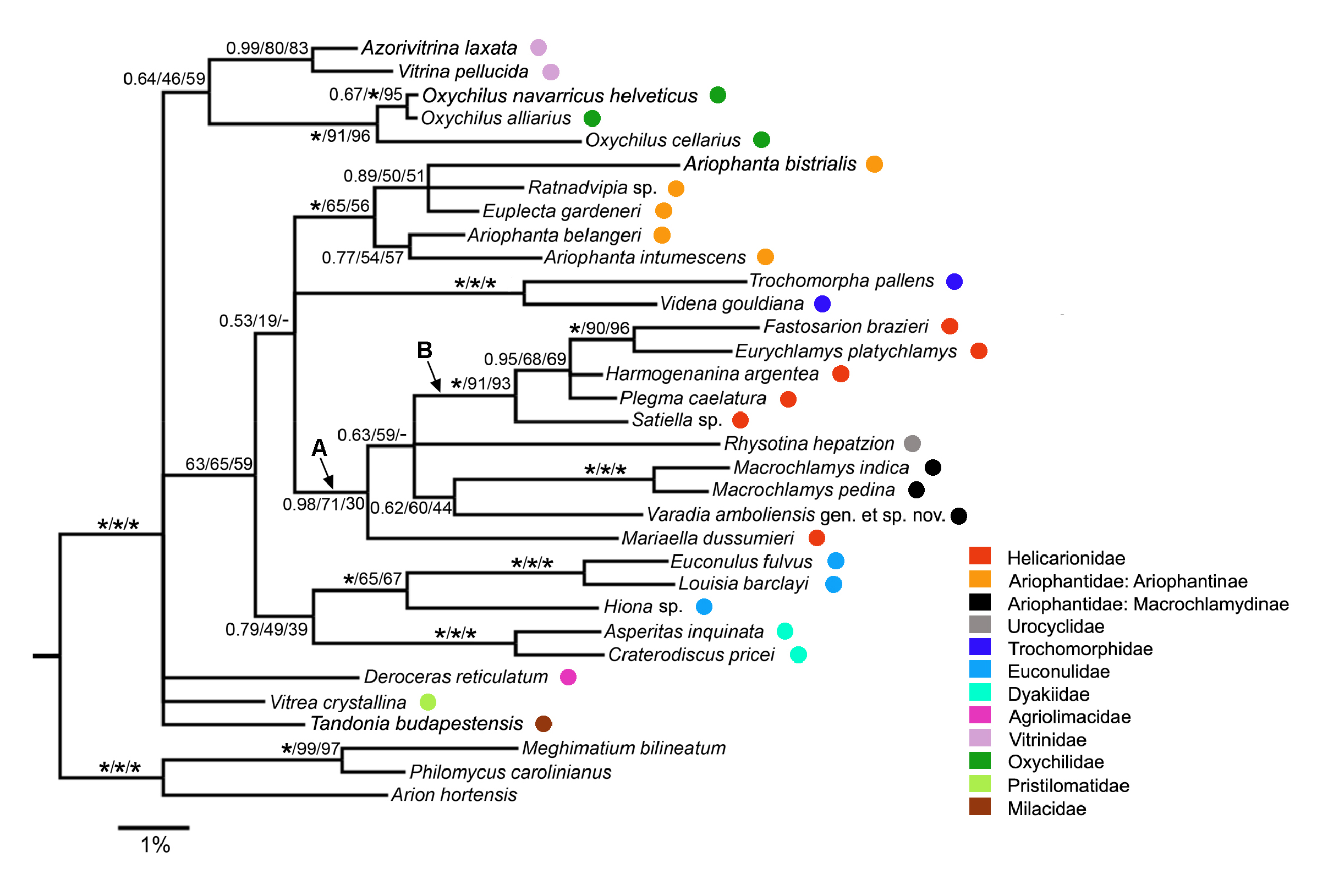
The three phylogenetic analyses (NJ, ML and Bayesian) yielded broadly similar results ( Fig. 14 View Fig ), with disagreements occurring only for internal branches lacking strong support in any of the analyses. Most deeper relationships within the Limacoidea were not strongly supported, with the optimal ML and NJ trees having fewer strongly supported branches than the Bayesian tree. All three trees included a sister- group relationship between Varadia gen. nov. and the always maximally supported Macrochlamys clade ( Macrochlamys indica Benson, 1883 + M. pedina (Benson, 1865)) , but this was not strongly supported in any of the analyses (Bayesian: PP = 0.62; ML: BS = 60%; NJ: BS = 44%). All analyses also provided maximal support for the clade composed of five of the six helicarionid taxa, Fastosarion brazieri (Cox, 1873) , Eurychlamys platychlamys (Blanford, 1880) , Harmogenanina argentea (Reeve, 1852) , Plegma caelatura (Férussac, 1821) and Satiella sp. (clade B in Fig. 14 View Fig ). Within clade B, support for the sistergroup relationship between Fastosarion Iredale, 1933 and Eurychlamys was consistently strong (ML: BS = 90%; NJ: BS = 96%) or maximal (Bayesian). Two of the three analyses (ML: BS = 71%; Bayesian: PP = 0.98) provided strong support for clade A, comprising clade B, Rhysotina hepatzion (Gould, 1848) , the Macrochlamys clade, Varadia gen. nov. and Mariaella dussumieri Gray, 1855 . All analyses provided maximal support for the monophyly of the Trochomorphidae Möllendorff, 1890 , of the Dyakiidae Gude & B.B. Woodward, 1921 and of the Euconulidae H.B. Baker, 1928 (i.e., clade comprising Euconulus fulvus (Müller, 1774) and Louisia barclayi (Benson, 1850)) . The monophyly of the Vitrinidae Fitzinger, 1833 and of the Oxychilidae Hesse, 1927 (1879) was also consistently strongly supported. Within the ingroup, nearly all the other branches were either strongly supported only in the Bayesian tree (e.g., clade comprising the five species of Ariophantinae sensu stricto) or were not strongly supported in any of the analyses.
Examination of all bipartition frequencies for the ML bootstrap trees (n = 1008) showed that the best supported bipartition that is not compatible with Varadia gen. nov. forming a clade with the two species of Macrochlamys is one in which Macrochlamys forms a clade with the five helicarionids, Fastosarion brazieri , Eurychlamys platychlamys , Harmogenanina argentea , Plegma caelatura and Satiella sp. (BS = 12%). Similarly, for the NJ bootstrap trees (n = 1000), the best supported bipartition that is incompatible with the clade of Varadia gen. nov. + Macrochlamys is the clade uniting all the helicarionids and Rhysotina hepatzion with Macrochlamys (BS = 14%).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |