Uvaria rovumae Deroin & Lötter, 2013
|
publication ID |
https://doi.org/ 10.5252/a2013n2a4 |
|
persistent identifier |
https://treatment.plazi.org/id/03F38786-FFA5-FFC9-FD25-F517C06103B4 |
|
treatment provided by |
Carolina |
|
scientific name |
Uvaria rovumae Deroin & Lötter |
| status |
sp. nov. |
Uvaria rovumae Deroin & Lötter , sp. nov.
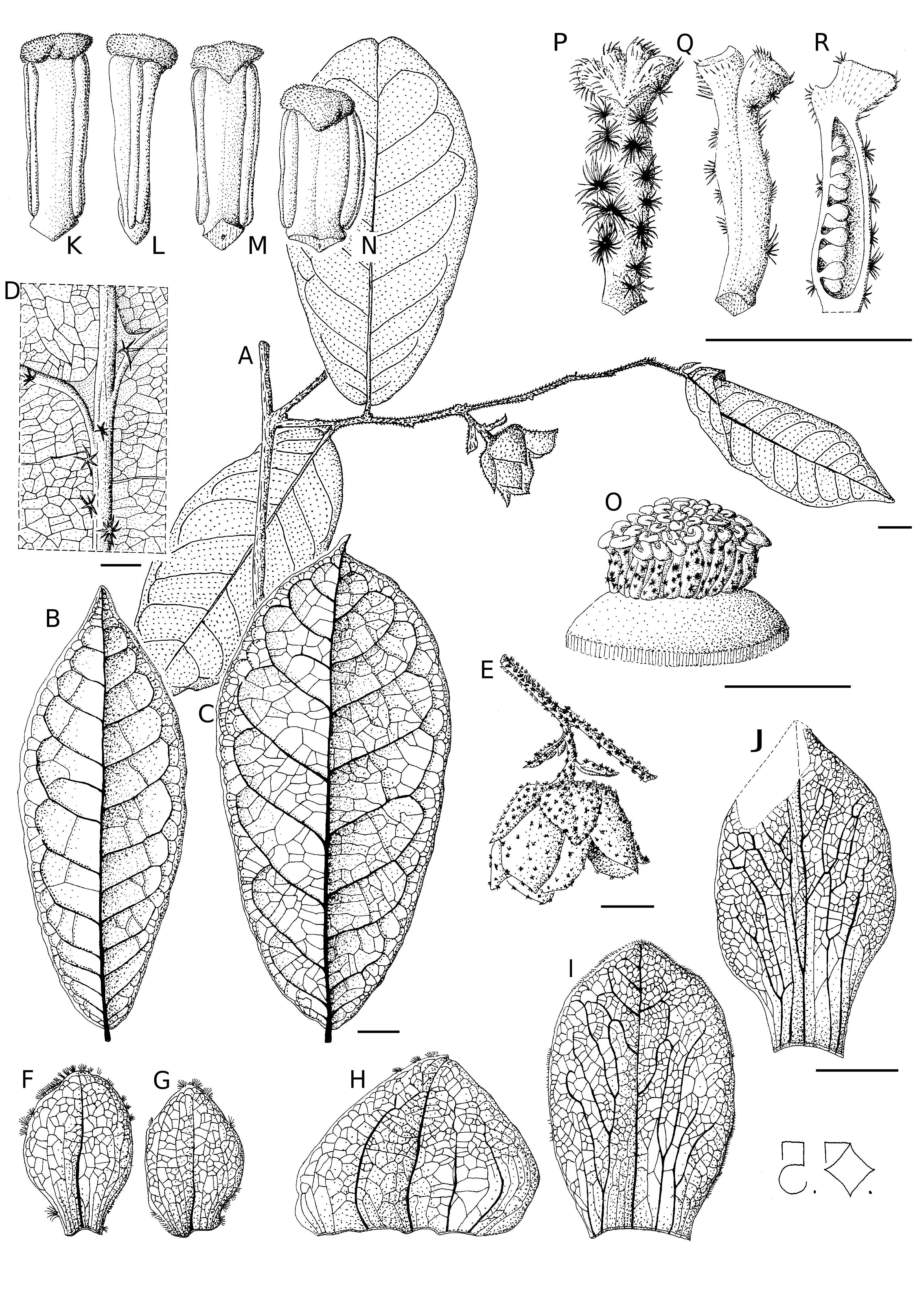
( Figs 1 View FIG ; 3 View FIG ; 5 View FIG )
Species habitu cum Uvaria tanzaniae Verdc. valde congruens, sed foliis non hirsutis subtus, pedicellis non obsoletis, calyce non cupuliformi sesquilatiori cum sepalis omnino liberis, petalis ellipticis vs. ovatis, neque tomentosis, plusminusve sesquilongioribus, intimis leviter unguiculatis, carpellis numerosissimis cum ovulis c. 10 vs 20 biserialibus, mericarpis nullo modo fusiformibus 2 vs.10-seminatis praecipue differt. TYPUS. — Mozambique. Cabo Delgado, Nangade , tall closed woodland, 11°12’05’’S, 39°38’48’’E, 332 m, 6. I GoogleMaps .2012, fl., Lötter 2142 (holo-, BNRH; iso-, K, P [ P00700917 ]!) .
PARATYPUS. — Mozambique. Cabo Delgado, 16 km south of Nangade , tall mixed closed woodland forest, 11°12.081’ S, 39°38.796’ E, 332 m, sandy soil, 23.III.2009, fl., Löt - ter & Turpin 1763 ( K, LMA, P [ P00700918 ]!) GoogleMaps .
ETYMOLOGY. — The specific epithet refers to the nearby Rovuma River.
DESCRIPTION
Scrambling liana c. 3-4 m tall, young branchlets rusty stellate-pubescent, later glabrous, pale brown, sparsely lenticellate. Leaves held in one plane; leafblades elliptic or obovate, 55-126 × 27-61 mm, obtuse acuminate or emarginate at the apex, rounded or somewhat cordate at the base, papery, densely pubescent when young, then glabrous above and beneath except for a few stellate hairs on the midrib and secondary veins (8-11 pairs), all veins slightly printed above, prominent beneath; petiole 3-6 mm long, stellate-pubescent.
Inflorescence leaf-opposed, peduncle c. 7 mm long, flowers 1(-2); bracteoles 2, c. 10 × 6.5 mm, pubescent outside, lower bracteole rounded slightly unguiculate, upper one elliptic. Torus as a depressed cylinder c. 14 mm in diameter, flat convex above. Perianth members basically with 5 primary nerves and a dense brochidodromous venation. Sepals broadly ovate, c. 11 × 15 mm, obtuse, free, pubescent outside, pale green. Petals subequal, gold yellow with a greenish tinge, apex obtuse or rounded, spreading, reflexed at the end of anthesis, the outer elliptic c. 18 × 11.5 mm, sparsely pubescent outside, the inner narrowly elliptic with a broad claw c. 20 × 11 mm, glabrous outside. Stamens numerous (c. 300, arranged in 6-7 whorls), linear c. 2 × 0.6 mm, latrorse, connective head truncate and minutely papillose, enlarged above narrow pollen sacs. The outermost stamens more rounded, c. 1.5 mm long. Carpels slender, c. 50, ovary c. 2 mm long, with scattered stellate hairs on the abaxial side, ovules c. 10, biseriate; stigmas horseshoe convoluted c. 0.5 mm thick, covered with simple hairs.
Fruit a pseudosyncarp ellipsoid in outline, c. 45 mm in diameter and 30 mm tall, pale yellow, borne on a peduncle c. 12 mm long and 3 mm thick, sepals persistent reflexed but not accrescent, mericarps c. 50 obovoid and apically rostrate, covered by sparse stellate hairs, 13-18 mm long, crowded on a globular receptacle by very short stipes (2 mm or less). Seeds 2 per mericarp, ellipsoid-irregular c. 6-8 × 4 × 4 mm, with a glossy reddish brown testa showing a weak pattern of endosperm rumination.
DISTRIBUTION, ECOLOGY AND PHENOLOGY Uvaria rovumae Deroin & Lötter , sp. nov., appears to be a range-restricted species, hitherto only recorded from the dense understory of a single large Newtonia paucijuga (Harms) Brenan tree, about 25 km south of the Rovuma River
near Nangade, 63 km north of Mueda ( Fig. 4). The surrounding vegetation was once probably tall semi-deciduous closed woodland or dry deciduous forest, but this has now largely been transformed to a mosaic of open to closed semideciduous woodland with isolated small patches of semi-deciduous forest. Forty-five years ago, Wild & Barbosa (1967) classified the vegetation in this area as Dry deciduous Lowland Forest. Our new species now only occurs in one of the very small remnant forest/thicket patches. Some of the associated woody species occurring with Uvaria rovumae Deroin & Lötter , sp. nov., include: Newtonia paucijuga, Bombax rhodognaphalon K.Schum. ex Engl., Millettia stuhlmannii Taub. , Strychnos myrtoides Gilg & Busse , Vismianthus punctatus Mildbr. , Whitfieldia orientalis Vollesen , Rinorea welwitschii (Oliv.)Kuntze subsp. tanzanica Grey-Wilson , and Streblus usambarensis (Engl.) C.C.Berg.
Floral biology ( Fig. 3 View FIG B-D) conforms to the pattern typical of Uvaria : no pollination chamber ( Saunders 2009: 578) and strict protogyny, with at first a receptive stigmatic plate covered by a mucilaginous cap ( Fig. 3B View FIG ) then a release of pollen grains ( Fig. 3D View FIG ).
CONSERVATION STATUS
After several botanical surveys in the region, this new species appears to be restricted to only one very small patch of forest undergrowth in an area to the north Nangade and just south of Mecabua village. This same locality harbours several other species of considerable biogeographic interest, such as two new records for Mozambique; Whitfieldia orientalis (Lötter & Turpin 1764) ; and Rinorea welwitschii subsp. tanzanica (Violaceae) (Burrows & Burrows 11314). It is also the second only locality of Streblus usambarensis (Moraceae) (Burrows & Burrows 11313) for Mozambique. An undescribed species of Lagynias E.Mey. (Rubiaceae) (Burrows & Burrows 11316) was also found in the same forest patch. The habitat of the first record of the Streblus usambarensis for Mozambique is classified by Wild & Barbosa (1967) as Dry Deciduous Lowland Forest, supporting our opinion that this area was once much more heavily forested.
The current site is dependent on the shade from a single large Newtonia paucijuga tree. The logging of this tree would allow light and fire to penetrate this biogeographically important forest patch. A large road running southwards from Nangade to Mueda cuts through part of this once larger forest patch. We suspect that more plants of Uvaria rovumae Deroin & Lötter , sp. nov., may eventually be found in the Rovuma River valley, or even in southern Tanzania. However it is currently only known from less than 5 individuals in an area no more than 30 × 30 m. Based on the IUCN Red List categories and criteria version 3.1 ( IUCN 2001), a provisional conservation status of Critically Endangered CR B1ab(iii); C1; D1 is thus proposed.
MORPHOLOGICAL REMARKS
The dissected flower of Uvaria rovumae Deroin & Lötter , sp. nov., showed two puzzling features:
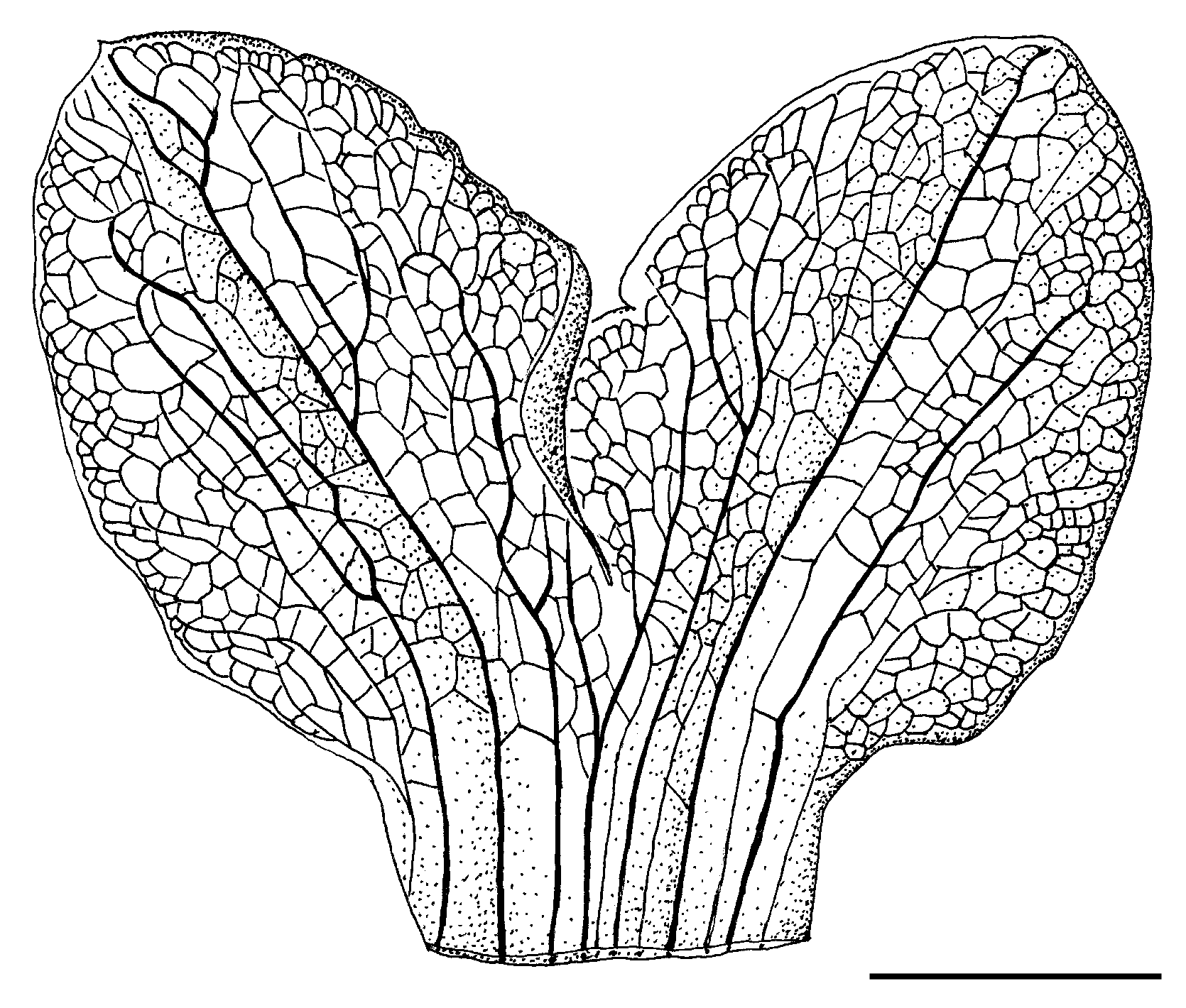
1) Two of the three inner petals were accidentally connate, as demonstrated by the merging of the
most lateral veins ( Fig. 2 View FIG ). A basal fusion of petals was previously reported in some species of the genus (van Heusden 1992: 150), but did not involve the primary vasculature.
2) Some of the most outer stamens are shorter and rounded ( Fig. 1N View FIG ), rather reminiscent of staminodes, frequent in Annonaceae ( Saunders 2009: 584) and usually present in several – especially Asiatic – Uvaria species (van Heusden 1992: 150), although they are here fertile.
Uvaria rovumae Deroin & Lötter , sp. nov. resembles members of the Uvaria angolensis group, as expanded by Johnson et al. (1999), in that its petals become strongly revolute during anthesis, but differs markedly by its free sepals and more numerous carpels (>35).
If the cupuliform calyx is not considered, U. rovumae appears intermediate between the two subgroups ( U. tanzaniae Verdc. + U. angolensis Oliver ) and ( U. lucida Bentham + U. puguensis D.M. Johnson ), being similar to the first in habit and petal size, much more to the second by the scattered pubescence of flowers. The similarity is striking with U. puguensis , especially in the torus morphology ( Johnson et al. 1999; Fig. 2E View FIG ) and petal colour (“yellow-green to pale orange-yellow”), as well as trichome, but the flower is much smaller (torus c. 5 vs 14 mm diam.), exhibiting a low stamen number (30-40 vs 300) with stretched quadrate vs truncate head connectives.
In their phylogenetic reconstruction of Uvaria, Zhou et al. (2012: 325) show that all African and Malagasy species are in a same clade II, splitting off during the Miocene, at c. 17.0 Ma. It is noteworthy that sepals are usually free or slightly connate at the base in Malagasy species ( Cavaco & Keraudren 1958: 7; Deroin & Gautier 2006), as in U. rovumae . A cupuliform calyx is a good character in an identification key, but cannot be used for outlining natural units. Perhaps is it possible to explain the free sepals of U. rovumae by a shift in the gene expression during floral morphogenesis ( Saunders 2009: 586). The synsepaly character, expected in this species-group, tends to be then expressed more inside in the perianth, while outer stamens are coming close to staminodes. It is possible that the increase of stamen and carpel numbers is correlated.
The pseudosyncarpic fruit ( Fig. 5 View FIG ) is similar to that of Uvaria scabrida Oliv. , a West African species ( Le Thomas 1969: 77). It is noticeable that in both species almost no carpel abortion occurs during fruit set. However in U. scabrida the floral receptacle is already globular, while in U. rovumae Deroin & Lötter , sp. nov. it appears flattened. On the other hand, if compared with the related U. tanzaniae Verdc. , only 20% of the ovules evolve in mature seeds and so the mericarps are not fusiform and even very slightly lomentaceous ( Verdcourt 1986: 287). These structural differences entail likely divergences in dispersal biology between these close species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |