Tricrania stansburii ( Haldeman), 1852
|
publication ID |
https://doi.org/ 10.5281/zenodo.200535 |
|
DOI |
https://doi.org/10.5281/zenodo.6194904 |
|
persistent identifier |
https://treatment.plazi.org/id/FE334E68-B673-A323-FF78-FA43B5C6882F |
|
treatment provided by |
Plazi |
|
scientific name |
Tricrania stansburii ( Haldeman), 1852 |
| status |
|
Tricrania stansburii ( Haldeman), 1852 : 377
Horia stansburii Haldeman 1852: 377 ; Lacordaire 1859: 664
Tricrania stansburii ; LeConte 1860: 320, new comb.; LeConte 1862: 270; LeConte 1869: 371; LeConte 1878: 472; Henshaw 1885: 130; Wellman 1910: 219; Hatch 1965: 116
Tricraniodes stansburii ; Wellman 1910: 219; Parker and Böving 1924: 30
Tricranioides stansburyi ; Borchmann 1917: 173
Tricraniodes stansburyi ; Leng 1920: 160; Stace Smith 1930: 23; Blackwelder 1939: 35
Tricrania stansburni ; VanDyke 1928: 403 [inferred synonomy with Tricranioides ]
Tricrania stanburyi ; Hatch 1950: 23
Tricrania stansburyi ; Linsley and MacSwain 1951; MacSwain 1956: 136; Gupta 1965: 446; Gupta 1971: 25; Erickson et al. 1976: 968; Gupta 1978: 149; Campbell 1991: 265; Torchio & Bosch 1992:713; Pinto & Bologna 1999: 608.
Tricrania murrayi LeConte 1860: 320 ; Henshaw 1885: 130; Borchmann 1917: 172; Leng 1920: 160; Blackwelder 1939: 35; Linsley & MacSwain 1951: 92; Hatch 1965: 116; Campbell 1991: 265 NEW SYNONOMY
Nomenclatural note. This species has been denoted by several names. The specific epithet was changed by Borchmann in 1917 from stansburii to stansburyi . Although the specific epithet honors Howard Stansbury, Haldeman used the spelling stansburii , thus, under Article 33.4 of the ICZN, stansburyi is an incorrect subsequent spelling and unavailable. No justification was given by Borchmann for the change. Following examination of the type of T.
murrayi and observations of the internal and external morphology of T. stansburii , we concluded that T. murrayi is indeed a synonym of T. stansburii .
Type material. Holotype— T. murrayi ( MCZ): 184; T. murrayi Lec. , Oregon; type 4951 [red label]
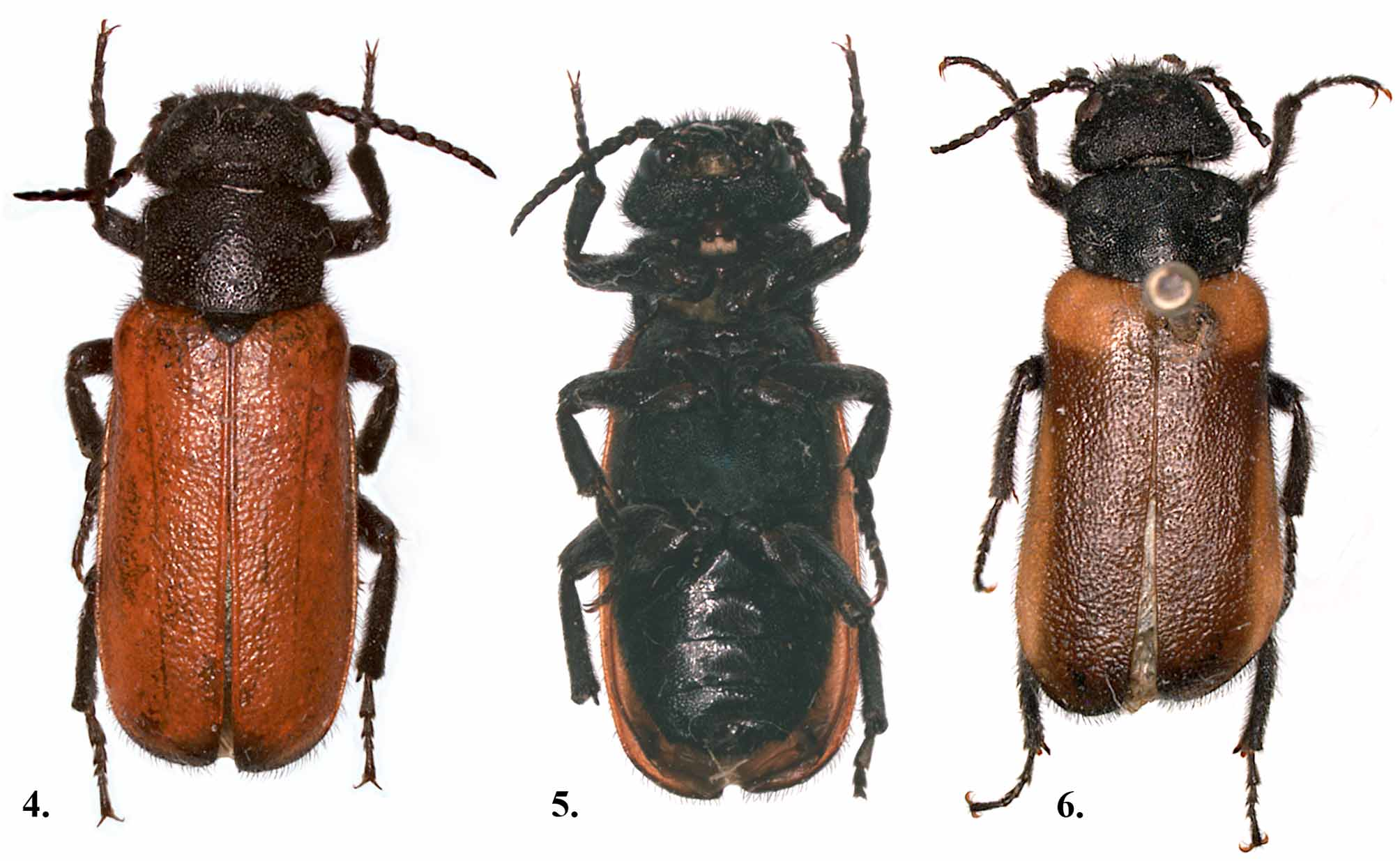
Adult redescription. Length: 8–14mm (mean = 12mm, N=20), width 3–6mm (mean = 4mm, N=20). Overall, body moderately convex; elongate. Body coloration black with brick-red elytra, head often with orange-red area on center of vertex, and apices of maxillary and labial palpi also orange-red; some specimens with variable dark maculations on elytra (see variation below). Body distinctly setose with stiff, erect black setae. Cuticular surface moderately to distinctly shining.
Head broadly triangular (W:L = 1.55:1); surface coarsely densely punctuate with irregular shaped punctures. Punctures ~3 diameters of eye facet; inner surface of puncture granular; each puncture gives rise medially to a single semi-erect or decumbent seta; interspaces between punctures narrow, <0.25 diameter of puncture and smooth to finely alutaceous in microsculpture; punctures not more widely separated on temples. Antenna elongate, antennomere comparative lengths as follows: 1.0: 0.63: 0.87: 0.63: 0.63: 0.56: 0.56: 0.56: 0.56: 0.56: 1.06. Terminal antennomere longest, less than length of antennomeres 9 and 10 combined, apex acuminate. Antennomeres 1 and 3 widest, antennomere 2 distinctly shorter and more narrow than 1 or 3.
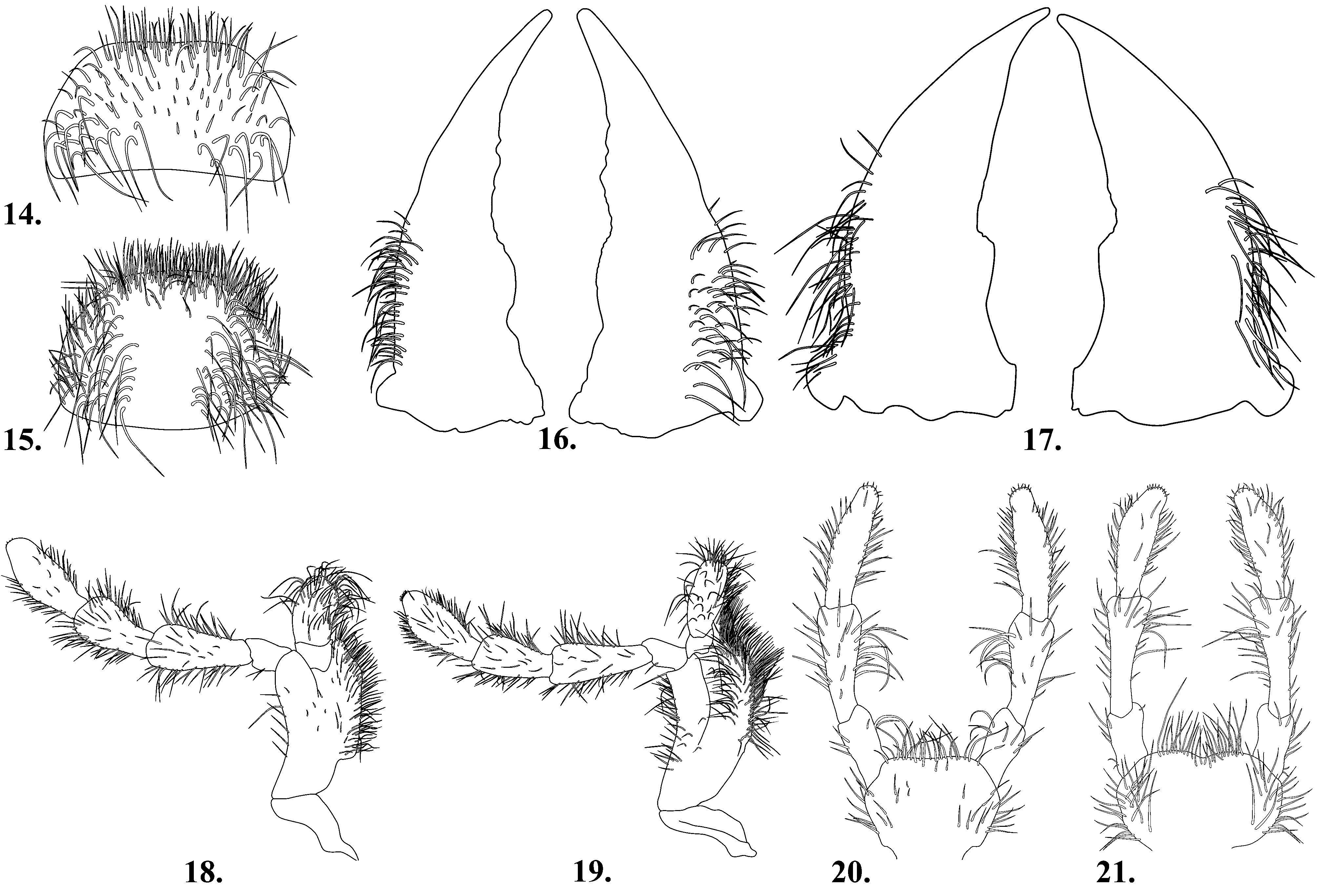
Mouthparts clearly visible, well developed. Labrum not slightly transverse, wider than long ( Fig. 15 View FIGURES 14 – 21 ); anterior margin appearing truncate to broadly convex, with dense setal brush; posterior margin broadly convex. Mandibles equally setose, each large, moderately robust, longer than wide; lateral region with numerous projecting elongate setae, ( Fig. 17 View FIGURES 14 – 21 ). Maxilla with terminal palpomere bearing numerous setae at apex; lacinia with lateral margin convex; galea with apex bluntly rounded ( Fig. 19 View FIGURES 14 – 21 ). Labium with anterior margin appearing emarginate; labial palpus elongate, terminal palpomere subequal to preceeding palpomere, apex with few scattered sort setae, distinctly swollen mesally ( Fig. 21 View FIGURES 14 – 21 ).
Pronotum transverse, appearing semi-rectangular, widest near anterior angles (W:L = 1.5:1); lateral margins faintly constricted in posterior 0.33; anterior and posterior angles broadly rounded; anterior and posterior margin broadly evenly rounded. Surface punctures similar to those on head; interspaces smooth to granular, shining, 0.25– 0.5 puncture diameters apart. Scutellum with apex bluntly rounded; deeply densely punctuate as on head and pronotum, but punctures somewhat smaller; interespaces finely granular to smooth, shining. Elytron with humeri moderately well developed; sutural striae not apparent along entire length; apices moderately to bluntly rounded. Surface punctures indistinct, appearing wrinkled with alutaceous to granular sculpturing. Prosternum deeply densely punctuate, punctures small, half the size of those on head or pronotum, often coalescing into transverse punctures or lines; interspaces smooth to granular, shining. Mesosternum with distinct medial glabrous region in anterior 0.33 of structure, surface smooth to granular; punctures on remaining areas similar to those on prosternum. Metasternum similarly punctate as pro- and mesosternum, sometimes with impunctate line medially, punctures more dense laterally; interspaces smooth, shining.
Legs elongate, well developed. Pro- and mesotibia with two unequal apical spurs, both spurs straight, anterior spur faintly bifid apically on protibia and trifid on mesotibia. Metatibial spurs unequal, both appearing flattened and spoon-shaped with the anterior spur somewhat shorter than posterior one. Tarsal claws complex, each claw with a ventral blade subequal to length of claw, each claw also finely denticulate from apex to 0.5–0.66 length of claw, not approximating base.
Ventrites 1–5 large, subequal in length to each other; punctures large and similar to those on head and pronotum, becoming more densely spaced laterally and often coalescing into each other; interspaces variable from smooth and granular, moderately shining.
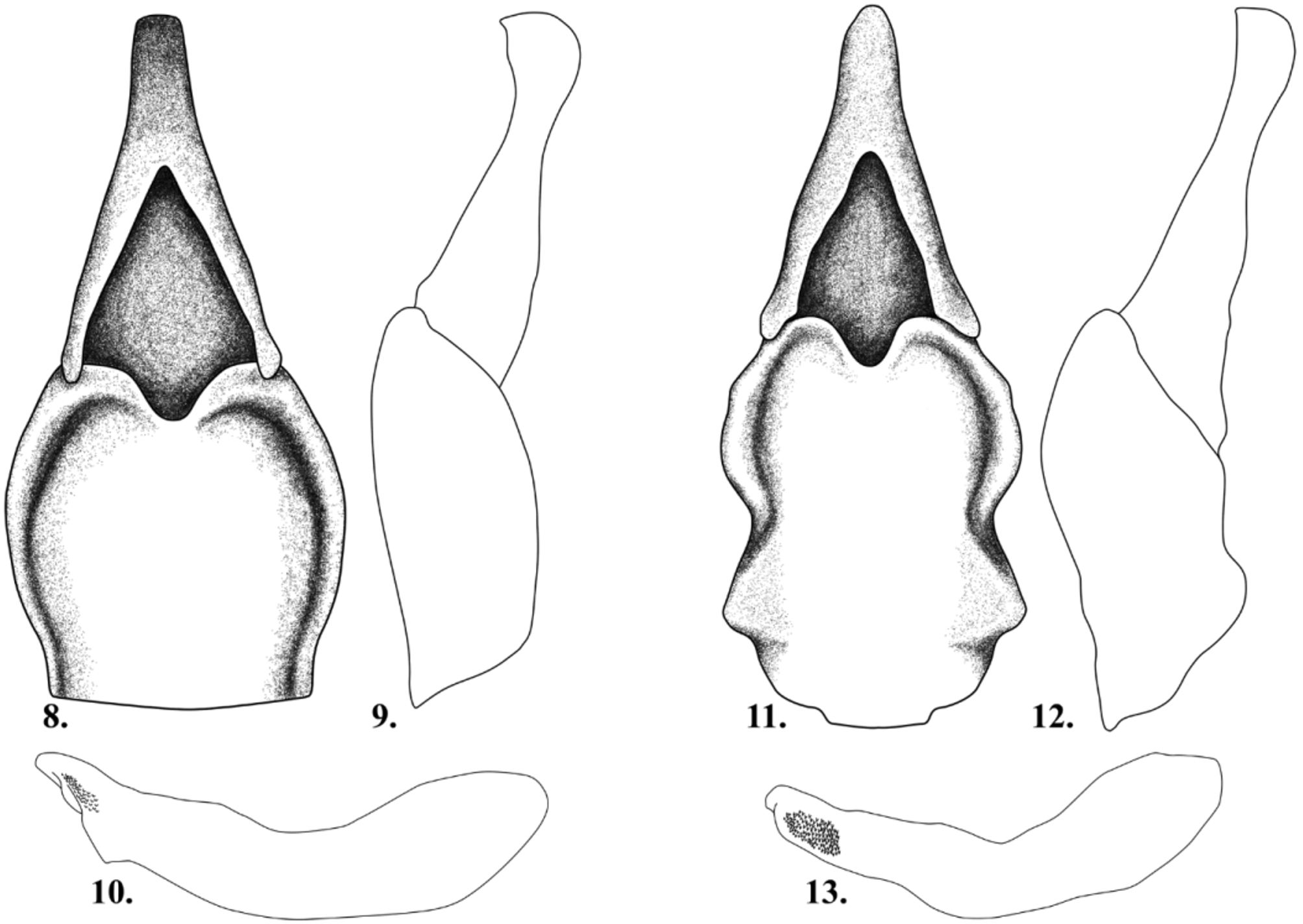
Male genitalia well sclerotized. Tegmen with parameres fused, and articulated with phallobase ( Figs. 11 View FIGURES 8 – 13 ). In ventral view, parameres evenly rounded apically, with deep medial fossa and deeply convex attachment to phallobase; phallobase wider than parameres, widest in posterior 0.33; middle with distinct medial constriction, median fossa broadly shallow. In lateral view, parameres with sharp apical point and phallobase not evenly convex ( Fig. 12 View FIGURES 8 – 13 ). Median lobe of aedeagus in lateral view with sharp declivity from apex to base, and large apical field of minute projections ( Fig. 13 View FIGURES 8 – 13 )
Female genitalia weakly to moderately sclerotized. Species level diagnostic character unapparent. As above.
Variation. Unlike the eastern T. sanguinipennis , this species exhibits marked color variation in the elytra ( Figs. 4, 6 View FIGURES 4 – 6 ). Most specimens collected possess elytra similar to that of T. sanguinipennis , i.e. uniformly dark brick red. However, several populations exhibit variable darkened areas on the elytra. Werner et al. (1966) noted that the elytral apices may be dark, but apparently only rarely in Arizona populations. We observed varying degrees of darkened elytra on specimens from southern California, Arizona, and New Mexico. The pronotal punctation and surface sculpture also is somewhat variable in several populations of this species, a fact that, in part, led to LeConte’s erection of T. murrayi , and the subsequent usage of that name. The number of tarsal denticulations also varies considerably. However, the male genitalia among several populations from throughout the range of T. stansburii indicate no significant differences and there was no geographic relationship between variation in color, pronotal punctation, and tarsal denticulation. These facts coupled with the known variability in color differences within other species of Nearctic Meloidae confirm the need for the synonomy of T. murrayi with T. stansburii .
Etymology. Specific epithet is a Latinized noun in the genitive case of Stansbury, the surname of the coordinator of the expedition into the Great Salt Lake Valley and presumably the person who collected the type specimen as well.
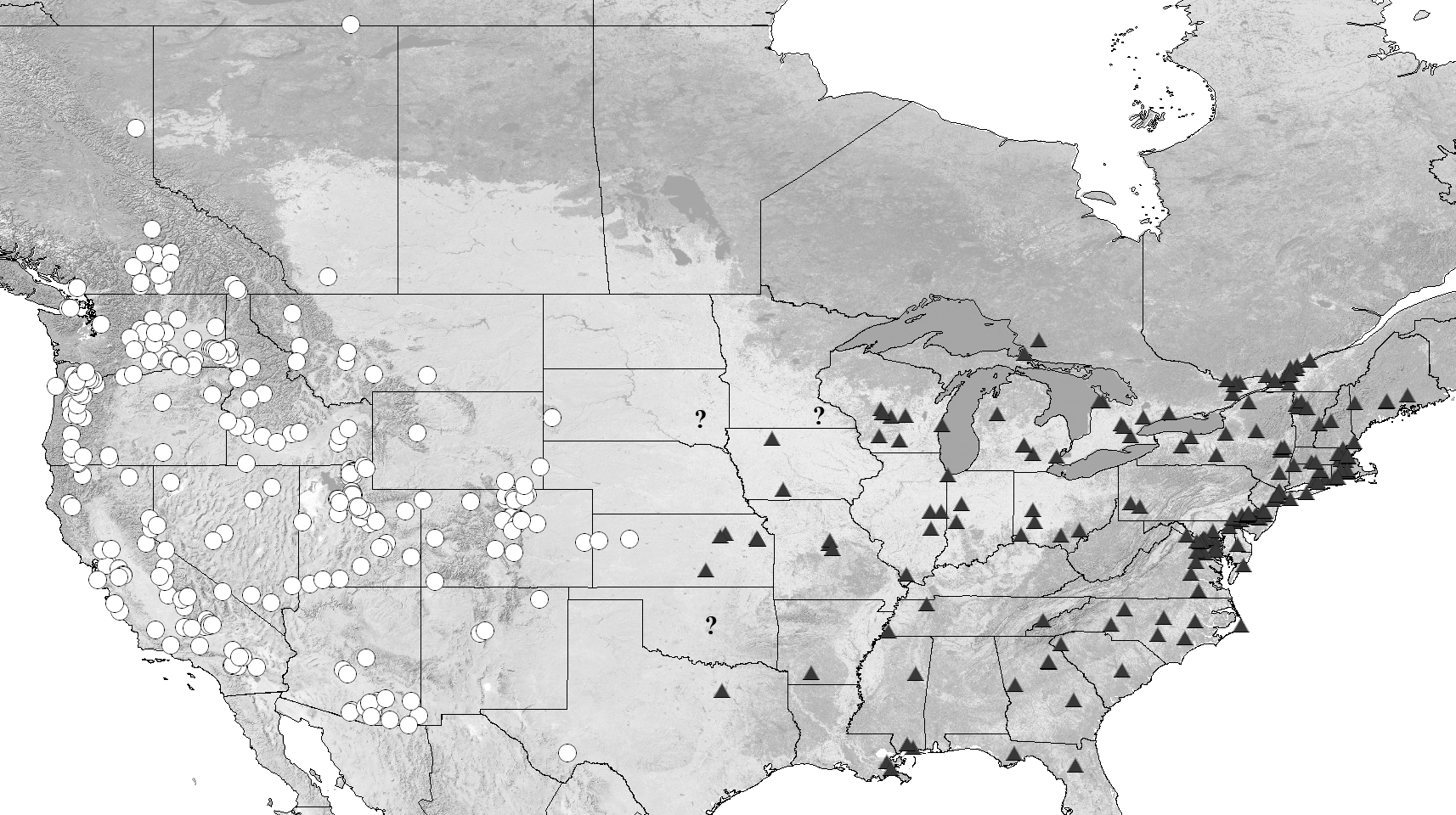
Distribution. Western and Central North America ( Fig. 30 View FIGURE 30 ), including: Canada (AB, BC, NWT) and the United States (AZ, CA, CO, ID, KS, MT, NE, NM, NV, OR, SD, TX, UT, WA, and WY). A record of T. stansburii in Nova Scotia, Canada by Majka et al. (2007) is undoubtedly an introduction event, as suggested by those authors, and no records prior to or subsequent to this event exist from that area. Therefore, this province of northeastern Canada has not been included in the distribution data listed here, or illustrated in the distribution map ( Fig. 30 View FIGURE 30 ). The above distribution is derived in part from our analysis as well as published records ( Hatch 1965 and Campbell 1991)
Biology. Linsley and MacSwain (1951) were the first to synthesize the known biology of T. stansburii ; however, Hicks (1926) recorded the species as a "parasite" from the nests of several bee genera (see Table 2 below). Werner et al. (1966) noted that adults are sluggish early in the spring and remain near bee nests they parasitize, and that they also do not visit blossoms; however, this appears unlikely based on the number and diversity of flowers upon which they have been collected (See Table 2 below), as well as the non-larval hosts from which triungulins have been acquired. Likewise they noted that this species had been reared from cells of Hoplitis , Osmia , Anthidium , and Anthophora ; and that males had been seen in flight. The latter was first proposed by Linsley and Mac- Swain (1951), who also noted never observing females in flight.
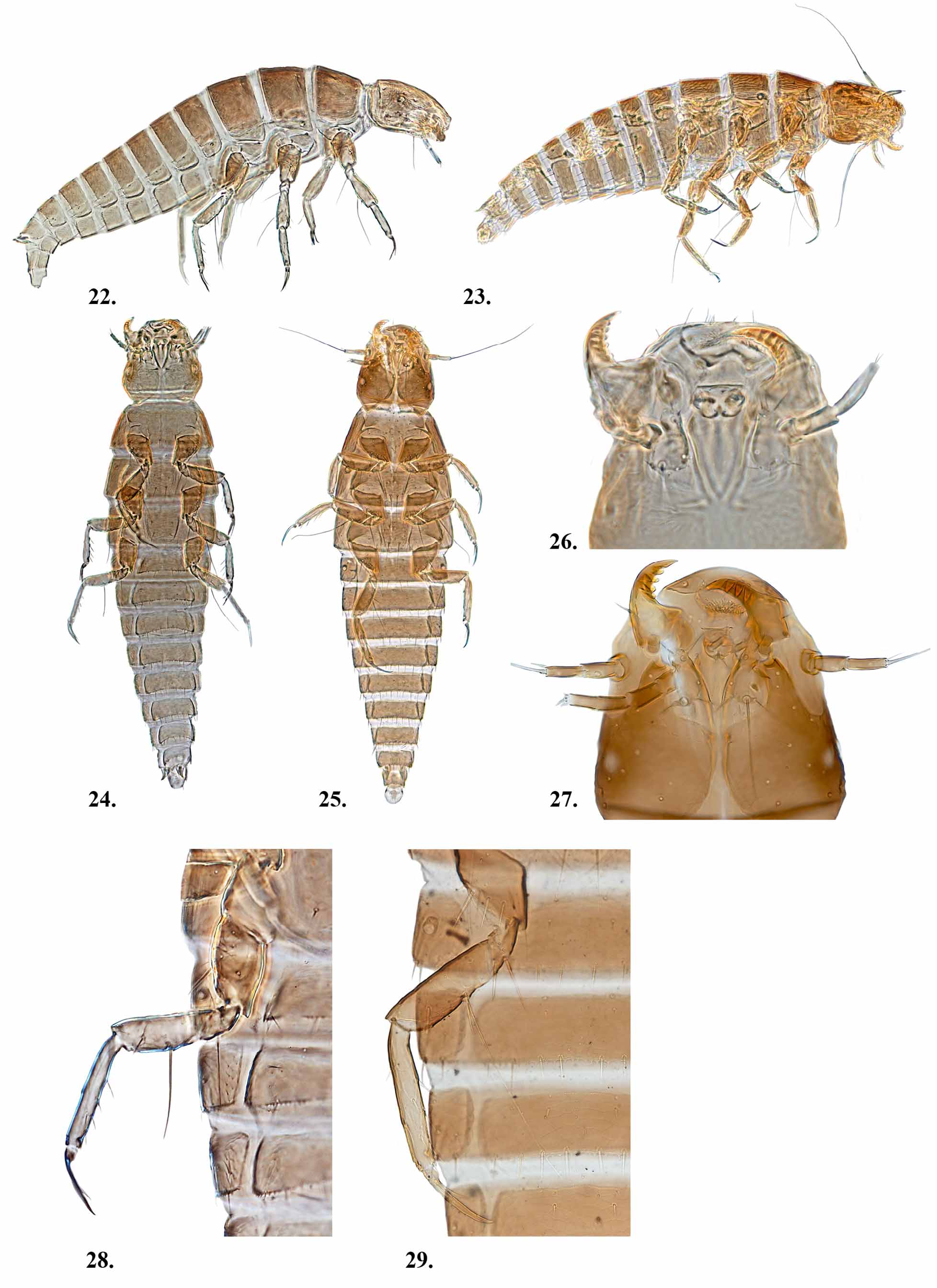
Notes. The first instar larval description and diagnosis has been previously completed in detail ( MacSwain 1956), and will not be repeated here; however illustrations are provided ( Figs. 23, 25, 27 View FIGURES 22 – 29 , & 29). We have included molecular sequence data, i.e. COI barcode region, in Appendix 4 as an initial step to promote the further explorations on the biology, systematics, and evolution of this species.
Tricrania stansburii , unlike T. sanguinipennis , exhibits variable elytral color pattern with black areas infringing upon and, in some extreme cases, almost engulfing the entire elytra. Enns (1956) rightly noted that intraspecific cuticular color variation is common among Nemognathinae species (i.e. many Nemognatha View in CoL , some Gnathium View in CoL , and also Zonitis View in CoL and Pseudozonitis View in CoL ), and stable patterns may even be rather uncommon (i.e. Nemognatha bifoveata ). A similar trend of low and high intraspecific variation also is present in pronotal punctation density in Tricrania View in CoL species. In T. sanguinipennis , pronotal punctation is fairly constant, whereas punctation is somewhat variable in T. stansburii . Enns (1956) also discussed the phenomenon of intraspecific punctation variance in both Zonitis atripennis and Z. cribricollis .
To better understand the causation of stable color patterns in one species and variable morphology in a sister species, we suggest a critical analysis and mapping of the natural history, phenology, geographic distribution, Hymenopteran host preference, and larval biology for Tricrania View in CoL as well as other Nemognathinae taxa via a robust cladistic analysis. Melanization of the cuticle is likely dependent on many external factors, but may also be attributed to evolutionary patterns that can be traced within a robust phylogenetic framework, whether that is at the population or species level. We did not detect any pattern of biogeographical pattern of elytral color pattern from the specimens examined.
Data from>550 unique collecting events for T. stansburii suggest a similar but slightly longer peak in adult activity as compared to T. sanguinipennis , beginning in late March and ending in late May. The peak in adult activity in T. stansburii occurs later in the season than T. sanguinipennis , with nearly as many recorded collecting events in May as in April, whereas in T. sanguinipennis there is a marked declivity in adult activity from April to May. The later shift of adult activity in T. stansburii may correspond to persistent cooler temperatures across its range, elevational effects from montane regions in western North America, and the later occurrence of some of the host bee genera parasitized by this species. Further studies are needed to support these hypotheses.
1 Hicks 1926, 2 Linsley & MacSwain 1951, 3 Werner et al 1966, 4 Torchio & Bosch 1992, authors pers. obs. or label data from
Appendix 3 below, 5slide mounted triungulins from EMEC and MTEC. *These records do not necessarily reflect rearing
records.
TABLE 2. Faunal, floral and miscellaneous collecting records for T. stansburii (Haldeman) *
| MCZ |
Museum of Comparative Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tricrania stansburii ( Haldeman), 1852
| Cline, Andrew R. & Huether, Jeffrey P. 2011 |
Tricrania stansburyi
| Pinto 1999: 608 |
| Torchio 1992: 713 |
| Campbell 1991: 265 |
| Gupta 1978: 149 |
| Erickson 1976: 968 |
| Gupta 1971: 25 |
| Gupta 1965: 446 |
| MacSwain 1956: 136 |
Tricrania stanburyi
| Hatch 1950: 23 |
stansburii
| Parker 1924: 30 |
Tricraniodes stansburyi
| Blackwelder 1939: 35 |
| Leng 1920: 160 |
Tricranioides stansburyi
| Borchmann 1917: 173 |
Tricrania stansburii
| Hatch 1965: 116 |
| Henshaw 1885: 130 |
| LeConte 1860: 320 |
Tricrania murrayi
| Campbell 1991: 265 |
| Hatch 1965: 116 |
| Linsley 1951: 92 |
| Blackwelder 1939: 35 |
| Leng 1920: 160 |
| Borchmann 1917: 172 |
| Henshaw 1885: 130 |
| LeConte 1860: 320 |
Tricrania stansburii (
| Haldeman 1852: 377 |
Horia stansburii
| Lacordaire 1859: 664 |
| Haldeman 1852: 377 |