Stylobatescalcifer, Yoshikawa & Izumi, 2022
|
publication ID |
https://doi.org/ 10.1086/719160 |
|
DOI |
https://doi.org/10.5281/zenodo.7573711 |
|
persistent identifier |
https://treatment.plazi.org/id/B955BB28-FFA0-7E47-FF79-F9A09F5F3C28 |
|
treatment provided by |
Tatiana |
|
scientific name |
Stylobatescalcifer |
| status |
sp. nov. |
Stylobatescalcifer View in CoL sp. nov. Yoshikawa and Izumi
[Japanese name: Hime-kin-kara-isoginchaku]
( Figs. 2–6, A2 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 )
Synonyms: Isadamsia sp. J : Uchidaand Soyama (2001), p. 81; Yanagi (2006), p. 141 (pl. I-I).
Stylobates sp. : Yoshikawa et al. (2019), p. 285 (fig. 1A); sea anemone ( Isadamsia sp. ): Jimi et al. (2021), p. 6 (fig. 1a, b, c).
ZooBank LSIDs (Life Science Identifiers): urn:lsid:zoobank.org:pub:D947FADF-9E1F-4136-A6A0-38227AAB5A32 .
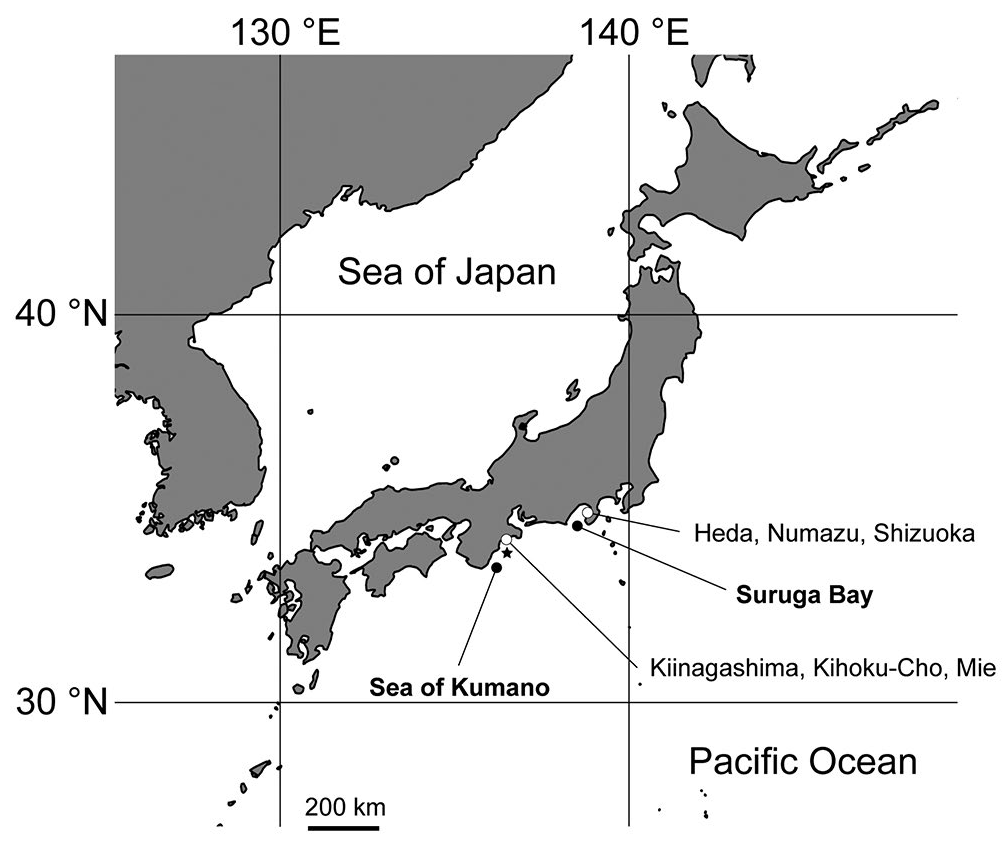
Material examined: Holotype: NSMT-Co 1794 , collected from the Sea of Kumano , off of the coast of the Kumano Region of the Kii Peninsula , Honshu , Japan (34°01, 10.0 ,, N, 136°23, 10.0 ,, E), by the bottom trawl net on the fishing vessel Jinsho-maru, on October 27, 2019, at a depth of 190– 300 m. GoogleMaps
Paratypes: CMNH-ZG 09764 , CMNH-ZG 09765 , CMNHZG09766 , CMNH-ZG 09772 , CMNH-ZG 09773 , CMNHZG09774 , CMNH-ZG 09775 , and CMNH-ZG 09776 , collected from the type locality at a depth of about 300 m; NSMT-Co 1795 and NSMT-Co 1796 , collected in a similar manner to NSMT-Co 1794 (34°01, 10.0 ,, N, 136°23, 10.0 ,, E) at a depth of 190–300 m; GoogleMaps CMNH-ZG 09774 , CMNH-ZG 09775 , and CMNH-ZG 09776 , collected from the type locality at a depth of about 300 m; CMNH-ZG 09767 , CMNH-ZG 09768 , CMNH-ZG 09769 , CMNH-ZG 09770 , and CMNH-ZG 09771 , collected from Sagami Bay , the coastof Heda , Numazu , Shizuoka, Japan, at a depth of about 300 m. All examined specimens are summarized in Table 1 View Table 1 .
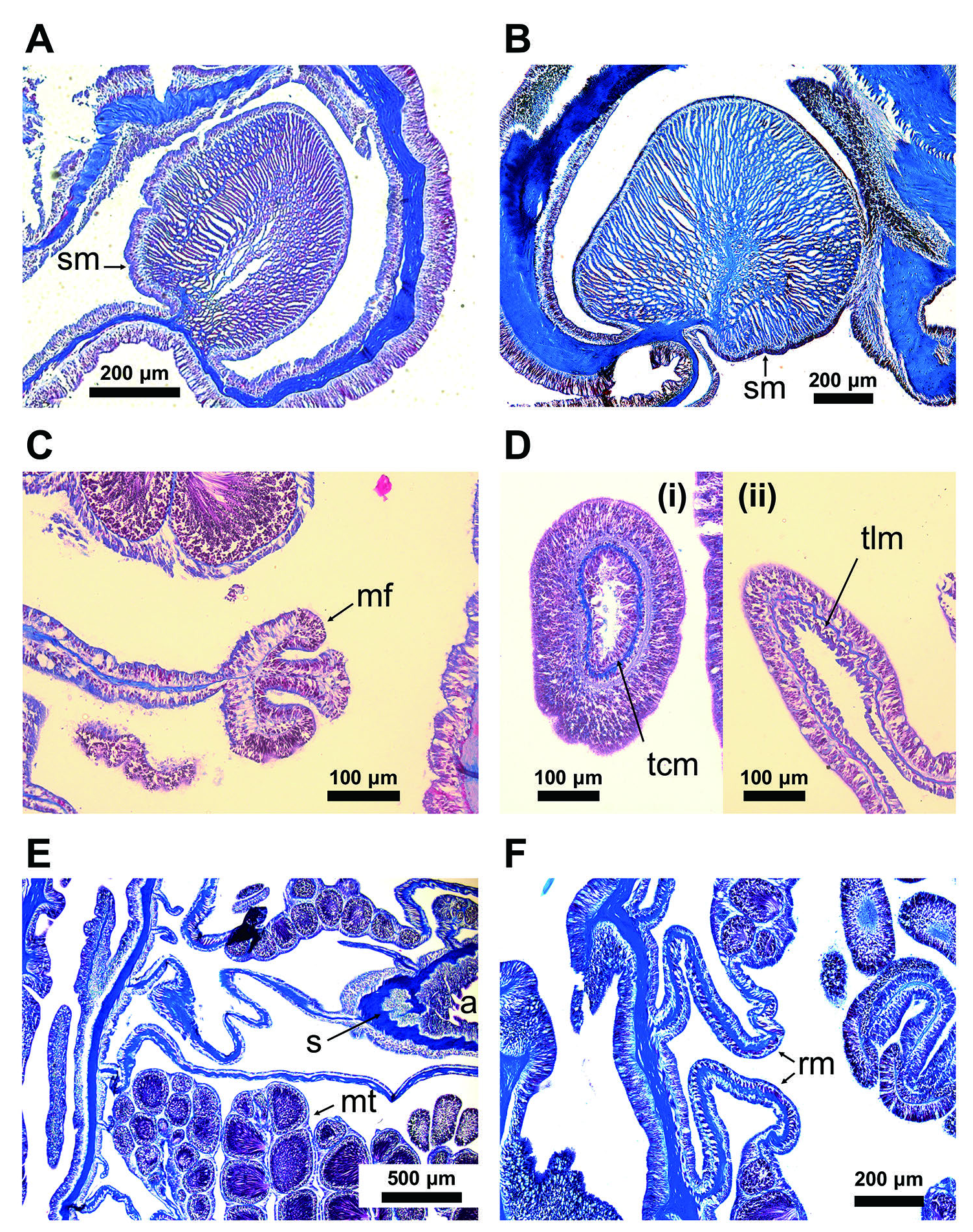
Morphology: Pedal disk thin, concave, conforming to the shape of the host shell. Diameter of the pedal disk depended on the shell’ s length and shape. Limbus thin, smooth with an irregular outline dependent on the shape of the shell, covering the entire shell except for the part under the hermit crab ( Fig. 2 View Figure 2 ). Column smooth, thin, flat, usually not cylindrical on the shells, with mesenterial insertions as dark lines ( Fig. 2B, F View Figure 2 ), 13.7–16.7 mm in height in fixed holotype, diameter flexible depending on the host shell’ s size and the sea anemone’ s conditions. Mesenterial arrangement biradial symmetry with its plane of symmetry perpendicular to the directive axis and its orthogonal axis. Oral disk flat with an oval mouth, 16.0– 20.7 mm in diameter in fixed holotype, with two prominent siphonoglyphs ( Figs. 2A View Figure 2 , 3E View Figure 3 , 4A, B View Figure 4 ). Mouth weakly swollen in the live animal ( Fig. 2C, D View Figure 2 ), flat in the preserved specimen ( Fig. 2E View Figure 2 ). Tentacles thin and pointed, inner ones 9.25–16.51 mm inlength and 1.19–2.62 mmin diameter, outer shorter than inner, 1.46–7.81 mm and 0.37–1.95 mm, respectively (in fixed holotype). The number of tentacles, 178 to 190 (178 in holotype) ( Table 1 View Table 1 ). The ectodermal musculature longitudinal in tentacles, radial in oral disks. Actinopharynx shallowly ribbed, with two symmetrical siphonoglyphs ( Figs. 3E View Figure 3 , 4A, B View Figure 4 ). Marginal sphincter muscle well developed, circumscribed, pinnate, with a short, radiate primary axis ( Fig. 3A, B View Figure 3 ).
Mesenterial arrangementin 96 pairsincyclesof 6, 6, 12, 24, and 48 ( Fig. 4 View Figure 4 ). Six pairs of the first cycle perfect, two pairs of the first mesentery directives each attached to siphonoglyph ( Fig. 4B View Figure 4 ). The third to fifth cycles with mesenterial filaments ( Fig. 4A, D View Figure 4 ). The fourthand fifth cycles fertile ( Figs. 3 View Figure 3 , 4 View Figure 4 ). Retractor muscles of mesenteries diffuse ( Figs. 3E, F View Figure 3 , 4B, C View Figure 4 ). Parieto-basilar muscle poorly developed ( Fig. 4D View Figure 4 ). Sexes separate, holotype male ( Fig. 3 View Figure 3 ), paratype (CMNH-ZG09775) female ( Fig. 4 View Figure 4 ). Mature spermatic vesicles 0.12–0.41 mm in diameter (holotype collected in November), mature oocytes 0.44–0.85 mm in diameter (paratype CMNH-ZG09775 collected in February). All of the developmental stages of the ova seen in a single mesentery.
Carcinoecium, varying from a thin chitinous coating on the gastropod shell to a coating with a shell extension and only extension of the shell aperture, thinner (1.0–5.0 mm) than the snail shell, dark brown without gloss ( Fig. 5 View Figure 5 ). The growth rings recognized on the surface; the layered structures absent, including dark-colored and white particles, fine sand, forams, and diatoms. Outer surface smoother and fewer exposed particles than the inner surface.
Cnidome: Spirocysts in the tentacle, basitrichs in the tentacle, actinopharynx, column, limbus, and microbasic b -mastigophores and p -mastigophores in the mesenterial filament ( Figs. 6 View Figure 6 , A 2 View Figure 2 , A 3 View Figure 3 ; Table 2 View Table 2 ).
Coloration in life: Base and column light red or pearl pink with dark mesenterial insertions ( Fig. 2A, B, C, D View Figure 2 ). Tentacles, oral disk, lips, actinopharynx, and siphonoglyphs with the same coloring ( Fig. 2A, C, D View Figure 2 ). Right after collection, coloration semitransparent, then turning whitish and somewhat opaque after several weeks in the aquarium.
Distribution and habitat: All of the samples were collected fromthe Seaof Kumano ( Uchidaand Soyama, 2001; Yoshikawa et al., 2019) and Suruga Bay. Allof the previousrecords were from the Pacific Ocean facing the middle of Honshu Island to Kyushu ( Uchida and Soyama, 2001; Yanagi, 2006). The specimens were distributed at 100–400 m from the fine sand and soft mud ( Fig. 1 View Figure 1 ; Table 1 View Table 1 ).
Ecological note: Stylobatescalcifer sp. nov. was exclusively found on the shells inhabited by Pagurodofleinia doederleini , consistent with previous studies ( Uchida and Soyama, 2001; Yanagi, 2006; Yoshikawa et al., 2019). However, P. doederleini without S. calcifer sp. nov. was sometimes collected in this study. One individual was usually attached to one host hermit crab, consistent with previous studies ( Uchida and Soyama, 2001; Yanagi, 2006; Yoshikawa et al., 2019).
Genetic analysis: In total, 6867 bp of 12S rDNA, 16S rDNA, 18S rDNA, 28S rDNA, and COIII genes were obtained from the 4 specimens of the newly identified species (CMNH-ZG09764, CMNH-ZG09765, CMNH-ZG09771, and NSMT-Co 1796). These sequences were compared to those of other Actinioideaspecies (DDBJ; Table A1 View Table 1 ) toexamine thephylogenetic relationshipsbetween Actinioidea . In the phylogenetic treeof thosethree regions ( Fig. 7 View Figure 7 ), Stylobates belongedtosuborder Enthemonae (node Aof Fig. 7 View Figure 7 ) and superfamily Actinioidea (node Bof Fig. 7 View Figure 7 ), with the ML bootstrap values/BI posterior probabilities of 100%/1 and 31%/1, respectively. All of the S. calcifer sp. nov. sequences formed a monophyletic clade supportedby 99% of the bootstrapvalues and oneposteriorprobability (node Cof Fig. 7 View Figure 7 ). Thesistercladeof S. calcifer sp. nov. was comprised of Stylobates loisetteae , with high support rate (node D) with ML bootstrap value/BI posterior probability of 99%/1. The interspecific variation of each sequence divergence wascalculated asfollows betweenthe 2 congeneric species in the phylogenetic tree: 0.000 in 12S, 0.002 in 16S, 0.094 in 18S, 0.281 in 28S, and 0.000 in COIII; the intraspecific variation was usually 0.000 or <0.004 ( Table A2 View Table 2 ).
Etymology: The specific name “calcifer” is driven fromthe name of a resident fire-demon Calcifer, which appeared in Howl’ s Moving Castle, a fantasy novel by British author Diana Wynne Jones, published in 1986; the story is the original version of the Japanese animated film of the same name, directedby Hayao Miyazaki (animated by Studio Ghibli). The shell-making ability of the new species in the species-specific relationship appears as if Calcifer was in a magical contract withthe Wizard Howl, constructing his Moving Castle.
Behavior: feeding response of Stylobates calcifer sp. nov.
Two feeding responses of S. calcifer sp. nov. (CMNHZG09774) were observed: the shrinking body (SB) type and the extending oral disk (EOD) type. An SB-type response occurred when the sea anemone obtained mashed foods. It began to shrink its sphincter muscles and opened its mouth widely immediately after the mashed foods fell on its mouth or tentacles (Video S1, available online). The SB-type response was recorded five times in this study. The EOD-type response occurredwhenthesea anemoneobtained krilland smallshrimps. Itextendeditsmouth inthe food’ s directionandslightlyshrank the marginal disk and tentacles to carry the food toward the mouth (Video S2, available online). The EOD-type response was recorded three times. In contrast, the sea anemone had no feeding responses to the skipjack tuna, brine shrimps, and liveshrimps (e.g., Palaemon spp. ) (Video S3, available online).
Behavioral relationship with host hermit crabs
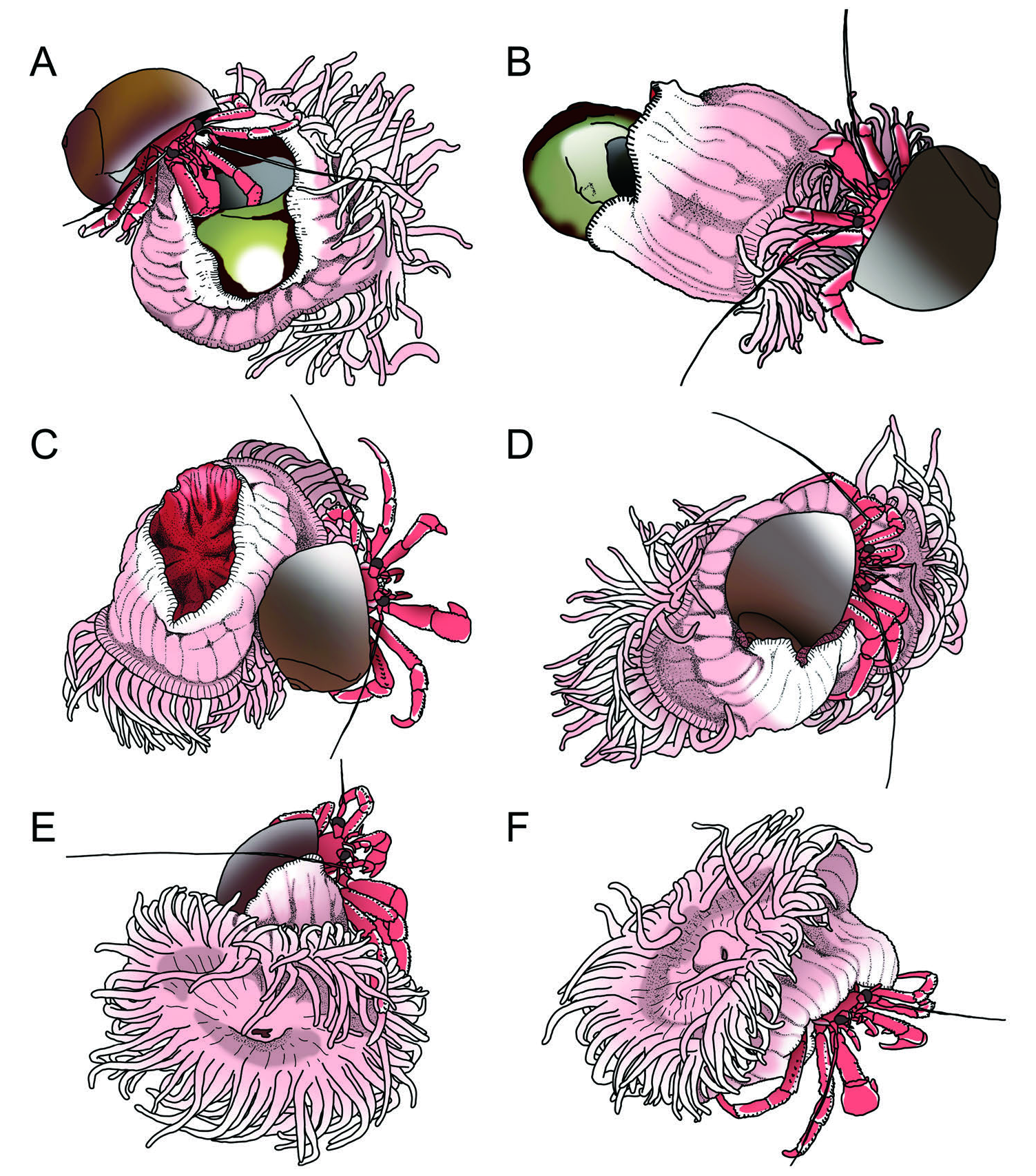
The first host hermit crab’ s shell change behavior was observed on February 20, 2020 (Video S4, available online). After the hermit crab entered the new shell, it walked to the empty shell and started to detach the sea anemone from the shell (observation S1: Video S4). The detaching behavior of the hermit crab consisted of short and quick tapping, pinching of the sea anemone with its walking legs and chelae, and massaging with its walking legs and its shell ( Fig. 8A View Figure 8 ; observation S1: Video S4). The hermit crab often tapped the edge of the pedal disk of the sea anemone and sometimes pinched the sea anemone’ s tentacles and oral disk, using both chelipeds. Moreover, the hermit crab rode on the sea anemone and shook and tapped it withthe shell rhythmically and constantly at about six stimulations per minute. Although the hermit crab had been continually giving the tactile stimulations to the sea anemone, there was no observed cooperative reaction, which was an apparent or quick reaction observed in other symbiotic sea anemones in shallow water (e.g., Calliactis spp. and Verrillactis sp. ) ( Ross, 1974, 1975; Yoshikawa et al., 2018). Then, the host hermit crab repeated the above behavioral series continuously; however, failure in transference of the sea anemone and its subsequent return to the previous shells are shown in observation S 1 in Figure A1 View Figure 1 .
The host hermit crab’ s second shell change behavior was observed on February 28, 2020 ( Fig. A1 View Figure 1 ). Although no apparent reaction was observed with this sea anemone, its position was gradually moved and peeled off from the shell ( Fig. 8B View Figure 8 ; observation S2-1: Video S5, available online). During the hermit crab’ s transference behavior, the column extended to the paniculate shape from the thin and flat shape (original shape) (observation S2-1: VideoS5). Finally, rather than being spontaneously detached, the sea anemone was peeled off from the shell by the hermitcrab about 12 hours after the crab’ s shell change (observation S2-2: Video S6, available online).
After detachment, despite the intense effort of the hermit crab to replace the sea anemone by carrying and fitting to the shell, the sea anemone showed no apparent shell-mounting action (observation S2-2: Video S6). However, the host hermit crab continued to carry the sea anemone and fit it onto the shell. When the sea anemone was turned to the upside-down position (facing up the pedal disk) ( Fig. 8C View Figure 8 ), the hermit crab rode on the pedal disk of the sea anemone and attempted to fit the shell to the curved line of the pedal disk ( Fig. 8C, D View Figure 8 ). The host hermitcrab kept trying until the sea anemone started shell mounting.
After about 43 h from the host’ s shell change and 18 h from detachment, the sea anemone began to mount the shell (observation S2-3: Video S7, available online). While the sea anemone was moving to the shell, the hermit crab was still tapping and pinching the sea anemone as in its detaching behavior ( Fig. 8E View Figure 8 ; observation S2-4: Video S8, available online). Finally, the sea anemone settled down on the new shell of the host hermit crab with a usual position facing the upper oral disk ( Fig. 8F View Figure 8 ; observation S2-4: Video S8). In summary, the transfer of the sea anemone took more than 24 h after the second shell change of the host hermit crab, that is, 12 h for detachment from the previous shell, 12 h for the mounting behavior after detachment, and 4 h to complete the transfer ( Fig. A1 View Figure 1 ).
In summary, the host hermit crab’ s shell change behavior was observed twice on February 20 (Video S4) and February 28, 2020. In the first trial (observation S1), the hermitcrab tried to transfer the CF anemone from 22:00 hours on February 20 to 24:00 hours on February 22 but failed, returning to the previous shell on February 22. However, in its second attempt (observations S2-1 to S2-4), it successfully transferred the CF anemone onto the new shell (observation S2-4: Video S8). Despite the persistent manipulation by the host hermit crab for more than 12 h, the sea anemone did not show a visible, consistent reaction to the crab.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Stylobatescalcifer
| Yoshikawa, Akihiro, Izumi, Takato, Moritaki, Takeya, Kimura, Taeko & Yanagi, Kensuke 2022 |
Stylobates sp.
| Jimi, N. & N. Hookabe & T. Moritaki & T. Kimura & S. Imura 2021: 6 |
| Yoshikawa, A. & S. Nakazawa & A. Asakura 2019: 285 |
Isadamsia sp. J
| Yanagi, K. 2006: 141 |
| Uchida, H. & I. Soyama 2001: 81 |