Schizophroida colemani, Ng & Ahyong, 2018
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.70.2018.1712 |
|
publication LSID |
lsid:zoobank.org:pub:AD2C3EEC-B184-43EA-8B98-52CD77D39B5A |
|
persistent identifier |
https://treatment.plazi.org/id/711F3E56-FFAA-F57B-3BE9-FDEFFA51FB34 |
|
treatment provided by |
Carolina |
|
scientific name |
Schizophroida colemani |
| status |
sp. nov. |
Schizophroida colemani View in CoL sp. nov.
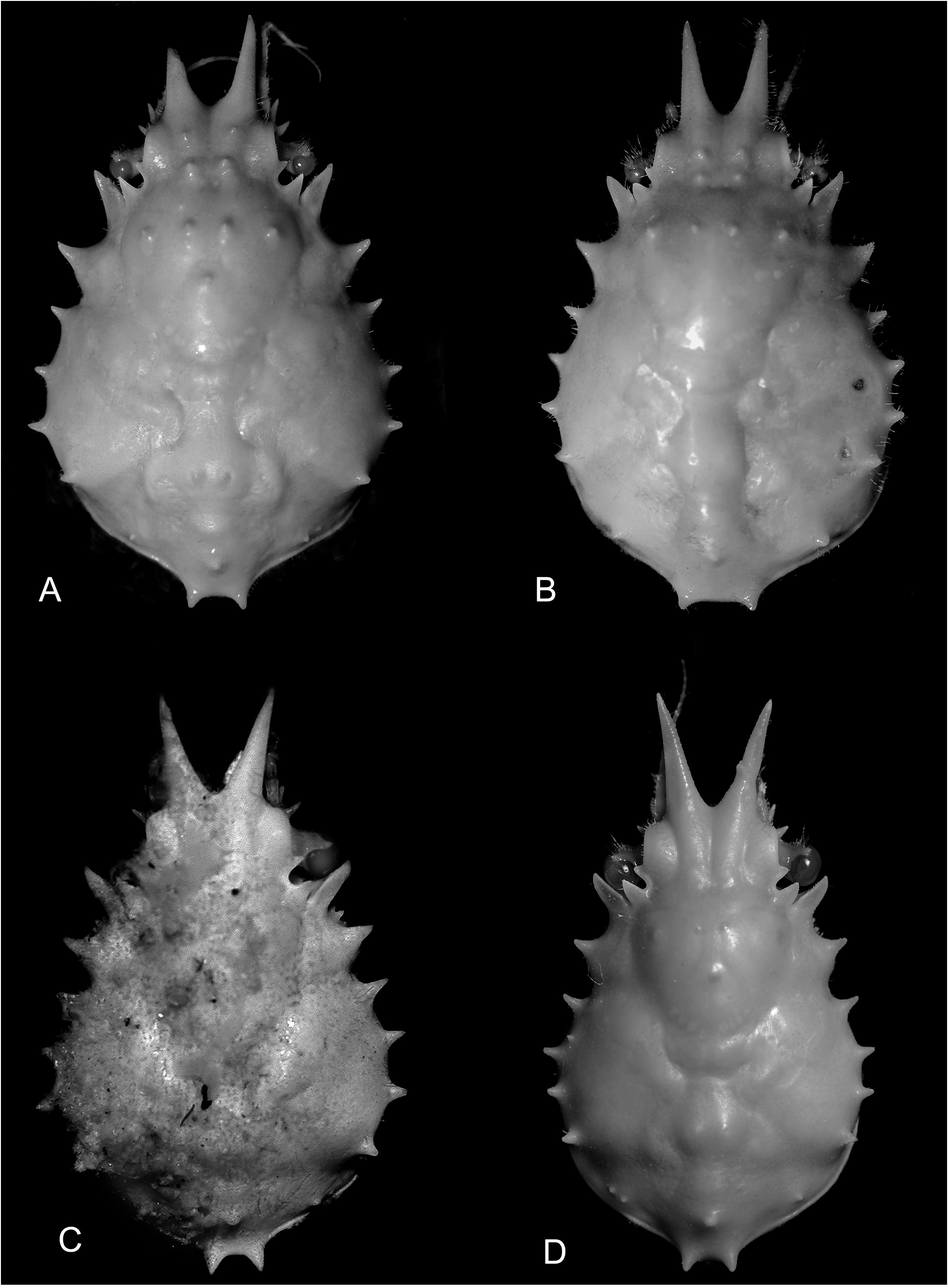
Figs 1 View Figure 1 A–C, 2A,B, 3, 5A, 10A–C
Schizophrys hilensis View in CoL .— ChiltOn, 1911: 546, 562–563.—DUffY & Ahyong, 2015: 83. [Not Schizophrys hilensis Rathbun, 1906 View in CoL ].
Schizophroida hilensis View in CoL .—Griffin & TrantEr, 1986: 238–243, fig. 68c, c, pl. 19 [part, AUstralian and NEw ZEaland specimens only].— Coleman, 2002: 56.— Poore, 2004: 380, fig. 114c.—TakEda & WEbbEr, 2006: 196, 232, fig. 3A.— Williams et al., 2008: 5.— Webber et al., 2010: 227.—Ahyong & Wilkens, 2011: tab. 1.—Yaldwyn & Webber, 2011: 239.— Ahyong, 2015: 429. [Not Schizophroida hilensis ( Rathbun, 1906) View in CoL ].
Holotype: AM P19606, male (cl 33.5 mm, pcl 27.9 mm, cw 20.2 mm), Ned’s Beach, coll. I. Bennett, May 1964 . Paratypes: AM P30966, 1 male (cl 18.4 mm, pcl 15.6 mm, cw 10.9 mm), 1 ovigerous female (cl 15.2 mm, pcl 13.5 mm, cw 9.2 mm), Deakon’s Reef, 22 m, coll. N. Coleman, 30 November 1979 ; ZRC 2017.1280 View Materials , 1 male (cl 18.0 mm, pcl 14.9 mm, cw 10.3 mm), Flat Rock, 25 m , AMPI Crust 866, coll. N. Coleman, 22 Feb 1979 ; AM P29818, 1 juvenile female (cl 9.9 mm, pcl 8.7 mm, cw 5.7 mm), Flat Rock, 25 m, coll. N. Coleman, 22 Feb 1979 ; AM P29819, 1 male (cl 19.4 mm, pcl 16.2 mm, cw 11.4 mm), Erscott’s Hole, 3 m , AMPI Crust No. 860, coll. N. Coleman, 15 February 1979 ; AM P80451, 1 male (cl 18.5 mm, pcl 15.4 mm, cw 10.3 mm), Erscott’s Hole, 4 m , AMPI Crust 954, coll. N. Coleman, 13 December 1979 . All Lord Howe Island .
Other material examined. AUSTRALIA.— NEW SOUTH WALES: AM P53496 1 ovigerous female (cl 26.8 mm, pcl 22.9 mm, cw 16.0 mm), between Jervis Bay and Gabo Island , NSW, 35°03'S 150°44'E, 30 m, coll. L. Vail GoogleMaps & V. Harriott , March 1981 .— LORD HOWE ISLAND, Tasman Sea : AM P29820, 1 juvenile female (cl 11.6 mm, pcl 9.8 mm, cw 6.8 mm), Ball’s Pyramid, 10 m , AMPI Crust No. 875, coll. N. Coleman, 24 February 1979 ; AM P10301, 2 juvenile females (cl 16.1 mm, pcl 14.0 mm, cw 10.0 mm; cl 20.3 mm, pcl 17.2 mm, cw 12.0 mm), nO spEcific lOcalitY ; AM P46665, 1 juvenile female (cl 13.3 mm, pcl 11.2 mm, cw 7.5 mm), Malabar, 15 m , AMPI Crust No. 1007, coll. N. Coleman, 23 February 1980 ; AM P5288 , 2 males (cl 9.0 mm, pcl 7.6 mm, cw 5.4 mm; cl 10.4 mm, pcl 8.8 mm, cw 6.3 mm), nO spEcific lOcalitY ; AM P29821, 1 malE (cl 9.0, pcl 7.5 mm, cw 5.3 mm), 1 juvenile female (cl 24.7 mm, pcl 21.2 mm, cw 14.9 mm), no spEcific lOcalitY, cOll. L. Clark, April 1932 .— TASMAN SEA: AM P39993, 1 male (cl 8.8 mm, pcl 6.9 mm, cw 4.7 mm), Elizabeth Reef , 29°57.8'S 159°04.7'E, outer slope,southern face, St. 36, coll. Australian Museum party, 11 December 1987 GoogleMaps ; AM P40007, 1 juvenile female (cl 8.8 mm, pcl 7.3 mm, cw 5.1 mm), Elizabeth Reef , 29°57.7'S 159°02.8'E, SW outer slope, St. 34, coll. Australian Museum party, 11 December 1987 GoogleMaps .
NEW ZEALAND. KERMADEC ISLANDS: AM P88937, 1 male (cl 3.7 mm, pcl 3.1 mm, cw 2.1 mm), Stawell Shoal , N of Stella Passage, 30°31.778'S 178°33.570'W, 21–24 m, under encrusting coral on rock, K2011-92-8, coll. S. Keable & A. Reid, 25 May 2011 GoogleMaps ; AM P88909, 3 males (cl 4.3, pcl 3.7 mm, cw 2.7 mm to cl 11.1 mm, pcl 8.9 mm, cw 6.4 mm), 4 females (cl 7.6 mm, pcl 6.0 mm, cw 4.6 mm to cl 14.2 mm, pcl 11.6 mm, cw 8.3 mm), W side l’Esperance Rock , 31°21.252'S 178°49.593'W, 12–20 m, rock walls, shelly sediment, sponges & coral scrapings, K2011-99-13, coll. S. Keable & A. Reid, 26 May 2011 GoogleMaps ; AM P89040, 1 male (cl 6.0 mm, pcl 4.9 mm, cw 3.6 mm), Fishing Rock landing, Raoul Island, 29°14.552'S 177°54.215'W, 5 m, K2011-49-1, scrapings from rock wall, 17 May 2011 GoogleMaps ; AM P89041, 1 juvenile male (cl 5.3 mm, pcl 4.3 mm, cw 3.1 mm), Dept of Conservation landing site, W side North Meyer Island, 29°14.674'S 177°52.688'W, 1 m, intertidal pools, K 2011-5-6 GoogleMaps , coll. T. Trnski , 12 May 2011 ; AM P89274, 4 males (cl 4.6 mm, pcl 4.1 mm, cw 2.9 mm to cl 13.0 mm, pcl 10.7 mm, cw 7.8 mm), 4 females (cl 7.1 mm, pcl 5.9 mm, cw 4.3 mm to cl 9.4 mm, pcl 8.1 mm, cw 6.0 mm), Fishing Rock landing, Raoul Island, 29°14.552'S 177°54.215'W, 5 m, K2011-49-4, scrapings from rock wall, 17 May 2011 GoogleMaps .— BAY OF ISLANDS, NORTH ISLAND: NIWA ( MITS) 21755, 1 ovigerous female (cl 27.1 mm, pcl 23.2 mm, cw 16.7 mm), frOm biOfOUling On flOating wrEck, BNZ1761 CB2 - CB, 10 March 2007 .
Diagnosis. Rostral spines subparallel, margins straight, shorter than 0.25 pcl. Carapace protogastric region with transverse row of 4 distinct tubercles in adults; posterolateral margin with subdorsal tubercle between posteromedian spines and posterior branchial marginal spine; marginal branchial spines and posteromedian spines straight. Supraorbital eave moderately broad transversely, anterior width narrower than half basal width of rostral spine; intercalated spine prominent, separated from posterior spine of supraorbital eve by narrow U-shaped sinus. Basal antennal article with inner distal spine longer than outer. G1 with distal one-third distinctly curving laterally to about 50° to longitudinal axis.
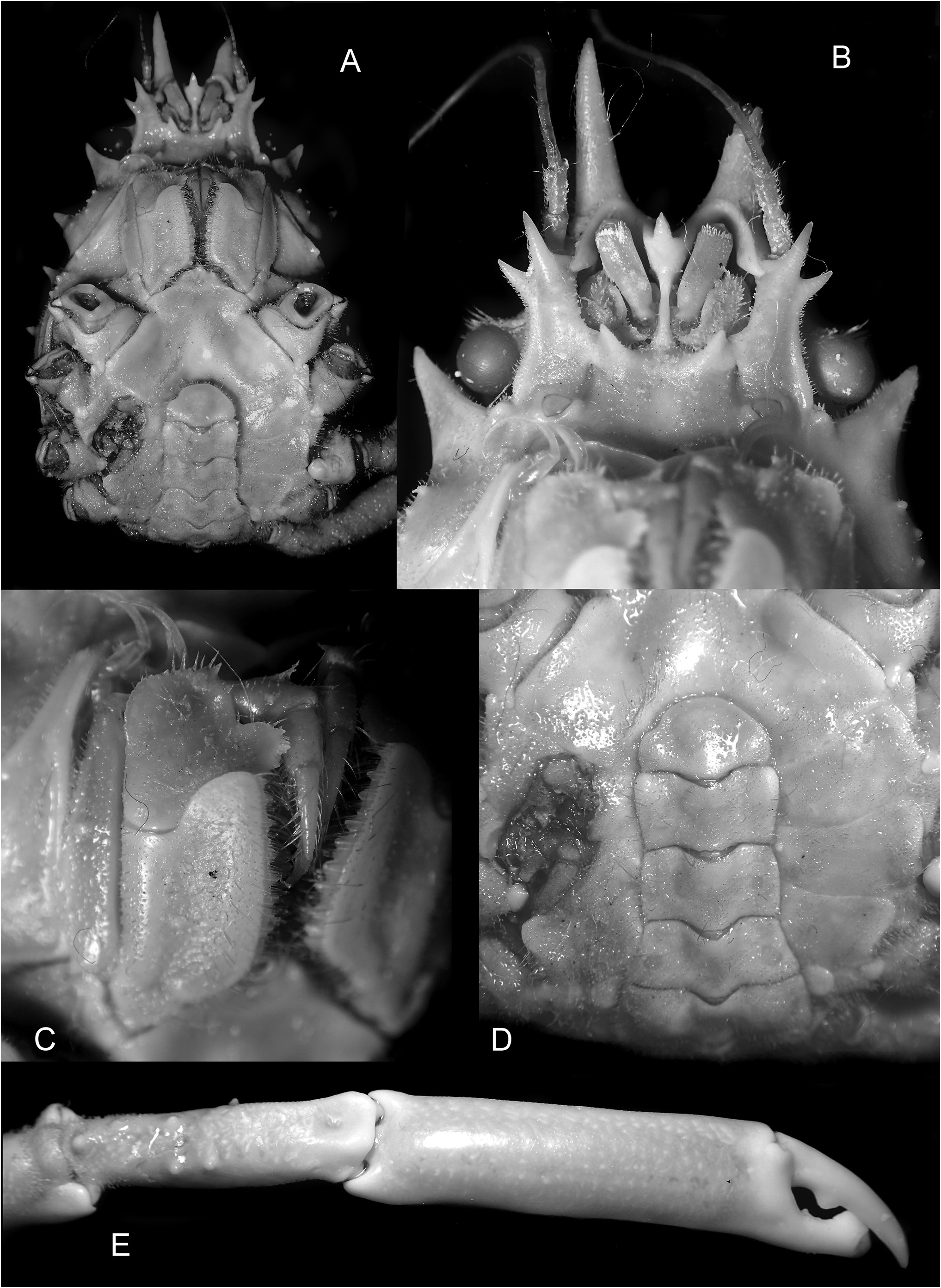
Description. Carapace ( Figs 1 View Figure 1 A–C, 2A, B) distinctly pyriform, longer than wide, regions weakly defined, surface densely covered with coarse straight and hooked setae. Rostral spines 0.13–0.22 pcl, margins straight, subparallel, occasionally slightly divergent in small juveniles. Supraorbital eave moderately broad transversely, anterior width narrower than half basal width of rostral spine; intercalated spine prominent, triangular, directed anterolaterally, separated from posterior spine of supraorbital eave by narrow U-shaped sinus. Carapace postfrontal region with longitudinal row of 2 tubercles behind each rostral spine; gastric region with 5 distinct tubercles: protogastric region with transverse row of 4 tubercles; mesogastric region with 1 tubercle; cardiac region with 2 low prominences; intestinal region with distinct median tubercle. Hepatic rEgiOn inflatEd; prOminEnt, cOnical, antErOlatErallY dirEctEd spine, larger than branchial spines; small subhepatic granules. Lateral branchial margin with 4 spines; posterior branchial margin with blunt subdorsal tubercle positioned midway between last branchial spine and paired posterior carapace spines. Posterior carapace spines straight, directed posteriorly, inclined slightly dorsad.
Epistome ( Fig. 3A, B View Figure 3 ) with stout anteroventrally directed spine at base of each antennular sinus. Interantennular septum cristate; interantennular spine directed ventrally, not visible in dorsal view ( Figs 2A, B View Figure 2 ). Basal antennal article distally bispinous, inner spine longer than outer spine.
Maxilliped 3 merus with rounded proximomesial lobe, margins usually with small denticles, distomesial margin with small spine; ischium distomesially auriculiform ( Fig. 3C View Figure 3 ).
Cheliped 1.29–1.90 pcl (adult males), 1.02–1.21 pcl (adult females); articles subcylindrical to subovate in cross-section. PrOpOdal palm smOOth, fingErs with gapE in adUlt malEs, pollex occlusal margin with blunt tooth near midlength. Dactylus slightly shorter than half palm length, occlusal margin with blunt tooth proximally. Carpus 2/3 palm length in adult males, with 6–8 rounded granules ( Figs 1A, B View Figure 1 , 3E View Figure 3 ); in females, smooth, unarmed, as long as palm.
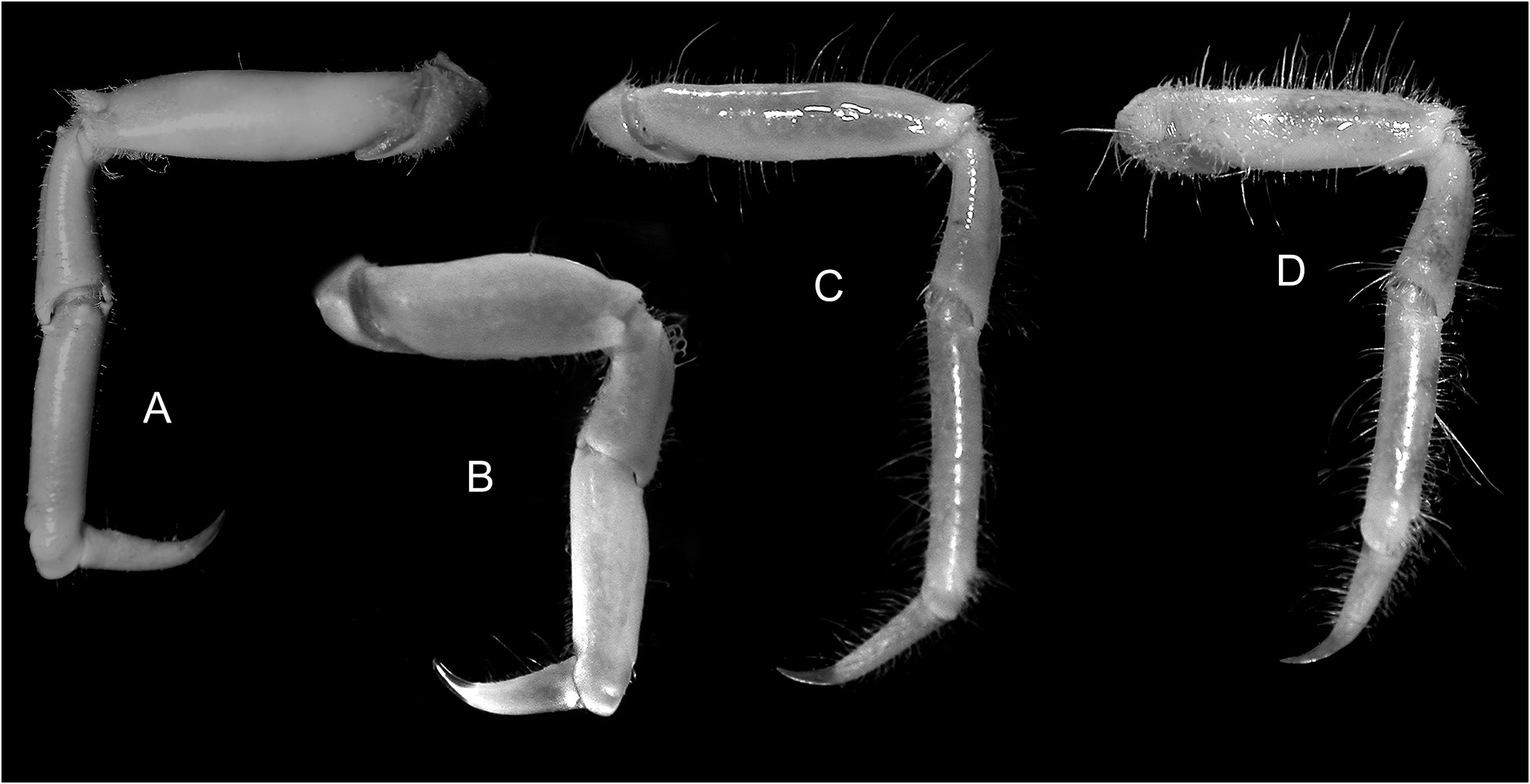
Pereopods 2–5 strongly setose, unarmed; dactylus simple, with curved, corneous tip. Pereopod 2 longest, merus 0.41–0.62 pcl (males), 0.42–0.46 pcl (females). Pereopod 5 merus 0.27–0.36 pcl (males), 0.28–0.32 pcl (females) ( Fig. 5A View Figure 5 ).
Male pleon with somite 4 trapezoidal, lateral margins weakly concave, margins converging distally; somites 5 and 6 subrectangular, margins weakly convex, widest at midlength and distal end, respectively; telson wider than long, distal margin evenly rounded ( Fig. 3D View Figure 3 ).
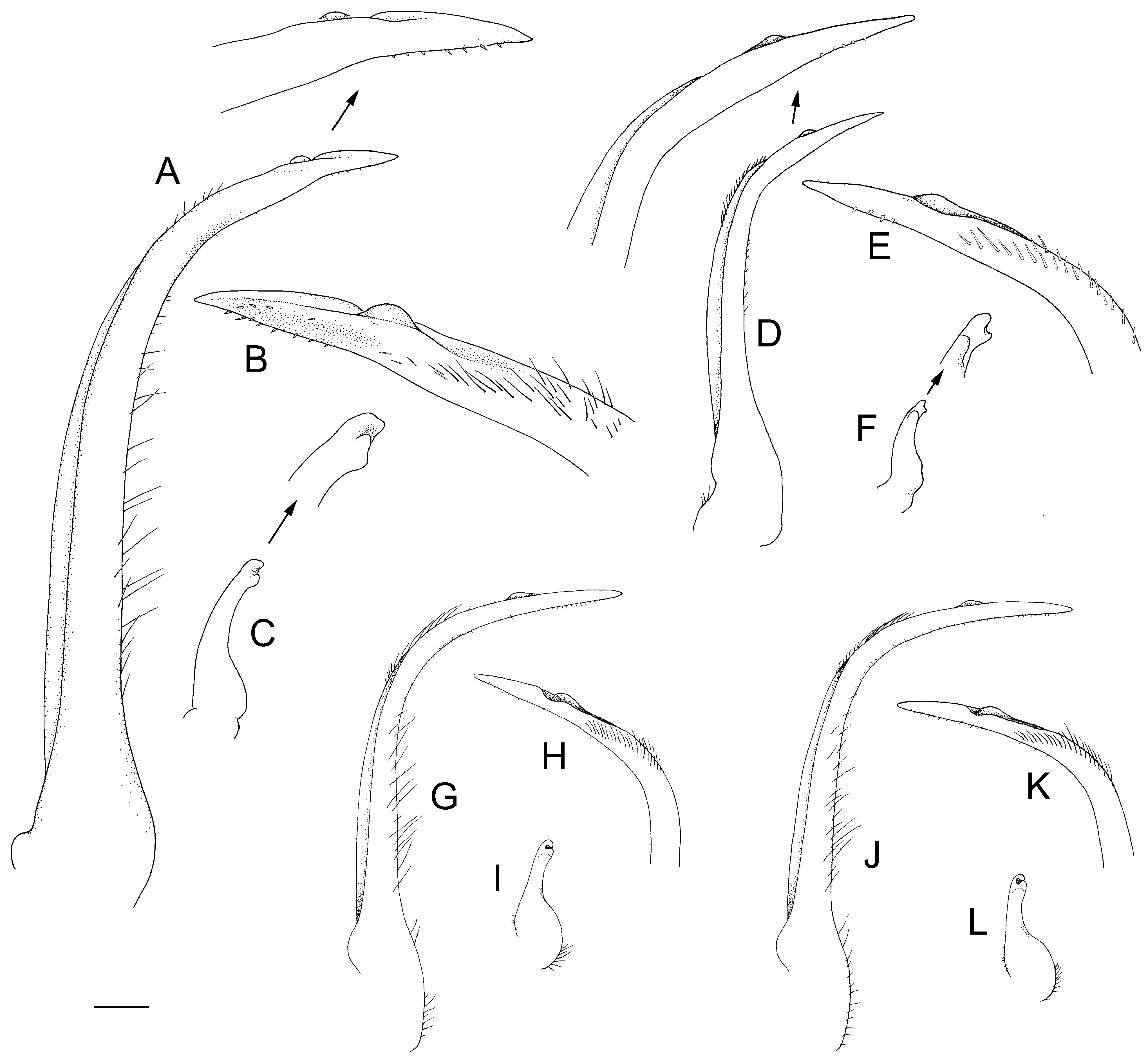
G1 long, slender, with distal one-third distinctly curving laterally to about 50° to longitudinal axis ( Fig. 10A, B View Figure 10 ). G2 simplE, distallY bilObEd, abOUt OnE-fifth lEngth Of G2, ExOpOd absent ( Fig. 10C View Figure 10 ).
Etymology. The species is named after the late Neville Coleman, who collected most of the specimens of the type series.
Remarks. Griffin & TrantEr (1986) nOtEd that spEcimEns of Schizophroida from Australia, New Zealand and New CalEdOnia diffErEd frOm Hawaiian matErial in thE dEgrEE of carapace tuberculation and the relative length of the basal antennal spines, although they considered them to be cOnspEcific. ThE prEsEnt rEsUlts indicatE that thE AUstralian and New Zealand population should be referred to a new species, herein named S. colemani sp. nov. The New CalEdOnian spEcimEn appEars tO rEprEsEnt a diffErEnt spEciEs again, discussed further below.As such, S. hilensis ( Figs 1D, E View Figure 1 , 2C View Figure 2 , 4 View Figure 4 , 5B View Figure 5 , 10 View Figure 10 D–F) is presently known with certainty only from the Hawaiian Islands.
In having a subdorsal posterolateral branchial tubercle on the carapace, S. colemani sp. nOv. diffErs frOm S. simodaensis but resembles S. hilensis , S. moai , and S. gracilis sp. nov. Distinctions between S. colemani and S. gracilis are discussed under the account of the latter. Schizophroida colemani resembles S. hilensis in the strong curvature of the G1 ( Fig. 10A, D View Figure 10 ), bUt diffErs in thE pOsitiOn Of thE distOmEsial lobe on the G1 (closer to the tip in S. colemani as in S. moai ), the lengths of the distal spines of the basal antennal segment (inner spine longer than outer in S. colemani versus EqUal in S. hilensis ; Figs 3B View Figure 3 , 4B View Figure 4 ), the narrower supraorbital eave (less than half basal rostral width versus exceeding half basal rostral width; Fig. 2 View Figure 2 A–C), in the presence of a transverse row of four prominent protogastic tubercles (at most two indistinct tubercles in S. hilensis ; Fig. 2 View Figure 2 A–C), in subparallel versus divergent rostral spines (occasionally subparallel in S. hilensis ; Figs 1 View Figure 1 , 2 View Figure 2 A–C), and a more evenly rounded male telson ( Figs 3D View Figure 3 , 4D View Figure 4 ). It should be noted that thEsE diffErEncEs arE bEst ObsErvEd in adUlts, in which thE carapace tubercles and other structures are fully developed. Schizophroida colemani resembles S. moai in the distinctness of the carapace tubercles and the length of the basal antennal spinEs, bUt diffErs in having parallEl instEad Of divErgEnt rostral spines, less pronounced carapace spines in which the anterior branchial marginal spines are shorter, rather than sUbEqUal tO hEpatic spinE, and thE pOstEriOr spinEs arE straight and slightly inclined dorsally, rather than upcurved. The male telson of S. moai is proportionally more elongate (Ng & BOYkO, 2017: fig. 13B vErsUs Fig. 3D View Figure 3 ) and thE G1 is also less strongly curved laterally than in S. colemani (Ng & BOYkO, 2017: fig. 17I, N vErsUs Fig. 10A View Figure 10 ).
Schizophroida colemani matures between about 10 and 13.5 mm pcl, with sexual dimorphism in the more elongated, robust male chelipeds evident by about 15 mm pcl ( Fig. 1 View Figure 1 A–C). The smallest ovigerous female examined here measured 13.5 mm pcl, though Takeda & Webber (2006) reported ovigerous specimens as small as 10.8 mm. Slight dimorphism is also evident in the length of the ambulatory legs as indicated by the proportionally longer pereopod 5 merus in adult males (0.33–0.36 pcl in males; 0.29–0.31 pcl females) ( Fig. 1 View Figure 1 A–C). Juvenile males, however, have pereopod proportions similar to those of females.
Allometric variation in S. colemani is EvidEnt chiEflY in the distinctness of the carapace tubercles, being most distinct in the largest specimens. The protogastric tubercles are usually absent in juveniles to about 10 mm pcl, and clearly evident by about 15 mm pcl. The protogastric tubercles can be indistinct in small specimens and are best observed by temporarily drying the surface of the gastric region. Similarly, the distinctness of the basal rostral tubercles is most pronounced in large specimens. The rostral spines are subparallel in all specimens except a few juveniles smaller than 10 mm pcl. In adult S. colemani , the outer basal antennal spine is shorter than the inner spine; however, the inner spine is initially the shorter in the smallest specimens (pcl 3.1–4.3 mm; apparEntlY first and sEcOnd crab stagE) bEcOming lOngEr with incrEasing bOdY sizE sUch that thE spinEs arE sUbEqUal by about 7 mm pcl and by about 9 mm pcl, the inner spine is longer than the outer spine.
As with many majoids, Schizophroida carries algal and OthEr biOfOUling as camOUflagE. An OvigErOUs fEmalE Of S. colemani (AM P53496) frOm Off sOUthErn NEw SOUth WalEs was found with three janiroidean isopods and one specimen of the amphipod Dulichiella cf. australis ( Haswell, 1879) (AM P53496) amongst the algal fouling on the carapace.
The northernmost records of S. colemani are from Lord Howe Island and the Kermadec Islands (~30– 31°S) and sOUthErnmOst rEcOrds frOm AUstralia arE Off sOUthErn New South Wales (35°S). In New Zealand, an ovigerous female was collected well south of its known natural range (KErmadEcs) amOngst biOfOUling On thE flOating wrEck Of a TaiwanEsE fishing vEssEl intErcEptEd in 2007 in thE BaY Of Islands, northeastern New Zealand ( Williams et al., 2008; as S. hilensis, NIWA (MITS) 21755). Schizophroida colemani is not known from mainland New Zealand; recruitment to the wreck probably occurred in the northern Tasman Sea in the vicinity of Lord Howe Island or the Kermadecs.
ThE spEcimEn frOm NEw CalEdOnia rEpOrtEd bY Griffin & Tranter (1986) (juvenile female pcl 15.2 mm, incomplete, lacking most pereopods, AM P29817; Fig. 3D View Figure 3 ) agrees with S. colemani in mOst rEspEcts bUt diffErs bY its distinctlY divErgEnt and more elongate rostral spines (0.25 versus 0.13–0.22 pcl) and evidently matures at a larger size ( S. colemani matures between 10.8 and 13.5 mm pcl). The protogastric tubercles are small and best observed when the carapace surface is dried. Additionally, the New Caledonian specimen was collected much further north than the northernmost S. colemani (Kermadec Islands) and from 85–100 m depth, considerably deeper than other species of the genus (intertidal to shallow sublittoral depths not exceeding 30 m). These bathymetric diffErEncEs maY bE Of significancE; dEspitE intEnsivE sampling, Schizophroida has not been recorded from deeper waters around northern New Zealand including the Kermadec Ridge ( Ahyong, 2008; Yaldwyn & Webber, 2011). The New Caledonian specimen probably represents an undescribed species, but is retained here as Schizophroida sp. pending collection of mature and more complete specimens.
Distribution. Coastal southeastern Australia, the Tasman Sea (Lord Howe Island, Elizabeth Reef) and the Kermadec Islands, New Zealand; intertidal rocky and coral reefs to 30 m.
| AM |
Australian Museum |
| V |
Royal British Columbia Museum - Herbarium |
| T |
Tavera, Department of Geology and Geophysics |
| NIWA |
National Institute of Water and Atmospheric Research |
| CB |
The CB Rhizobium Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Schizophroida colemani
| Ng, Peter K. L. & Ahyong, Shane T. 2018 |
Schizophrys hilensis
| Ahyong, S 2015: 83 |
| Chilton, C 1911: 546 |