Rhabdopleura emancipata, Gordon & Randolph Quek & Huang, 2024
|
publication ID |
https://doi.org/10.11646/zootaxa.5424.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:524CF65D-F877-42E1-B983-EDC7D3ED1623 |
|
DOI |
https://doi.org/10.5281/zenodo.10821357 |
|
persistent identifier |
https://treatment.plazi.org/id/0381104D-FFDD-B94E-EAF0-FE10F139FD2A |
|
treatment provided by |
Plazi |
|
scientific name |
Rhabdopleura emancipata |
| status |
sp. nov. |
Rhabdopleura emancipata n. sp.
( Figs 2C View FIGURE 2 , 11A‒E View FIGURE 11 , 12A‒E View FIGURE 12 , 13A‒H View FIGURE 13 )
Material examined. Holotype: NIWA 13590 View Materials , NIWA Stn E861, Wanganella Bank , near West Norfolk Ridge, 32.4167° S, 167.5833° E, 318 m, unattached, 18 March 1968, in ethanol GoogleMaps . Paratype 1: NIWA 162566 View Materials , same station data as holotype, dried colony fragments GoogleMaps . Paratype 2: NIWA 161211 View Materials , glycerol slide mount of small fragment of holotype .
Etymology. Latin emancipatus, set free, alluding to the unattached sole colony.
Diagnosis. Mature colony unattached, three-dimensional, erect and tangled, c. 4.5 cm diameter, erect ringed tubes budded directly from surface of principal tubes (stems) at any angle; stems lacking zigzag sutures. Pectocaulus varying in position along stems. Erect tubes spaced 309‒521 μm apart, mean erect-tube diameter 178 μm, mean fusellus height 31 μm. Darkly sclerotized tubes occurring sporadically on stems, slightly tapered proximally and distally.
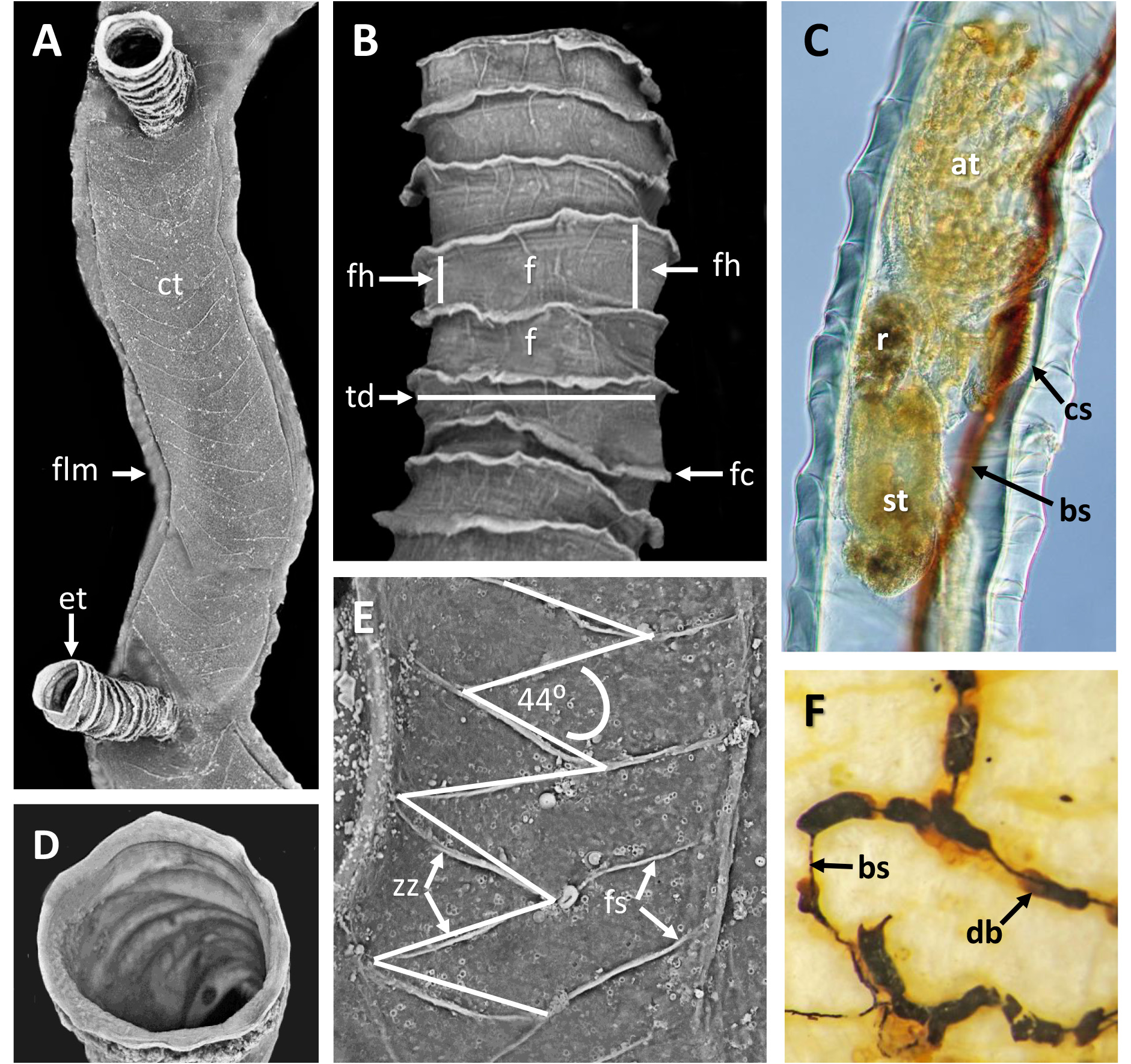
Description. Sole colony not attached to any substratum ( Fig. 11A, B View FIGURE 11 ), relatively large, forming ~ 4.5 cm diameter ‘ball’ of branching tubes in small jar of ethanol, flattening to 6 cm in petri dish for microscopic examination. No obvious colony attachment point, but many tubes trending in a similar direction, otherwise tangled.
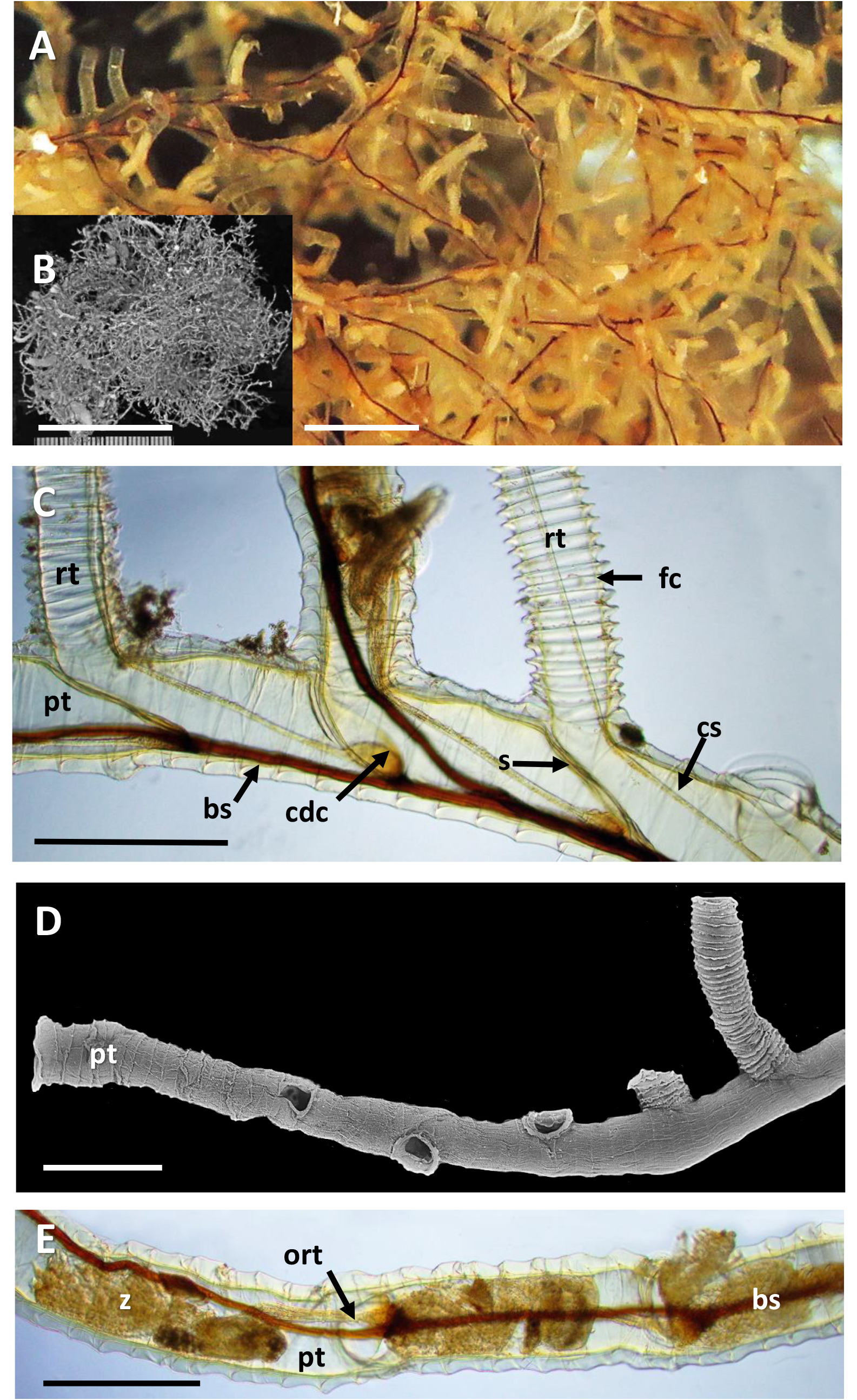
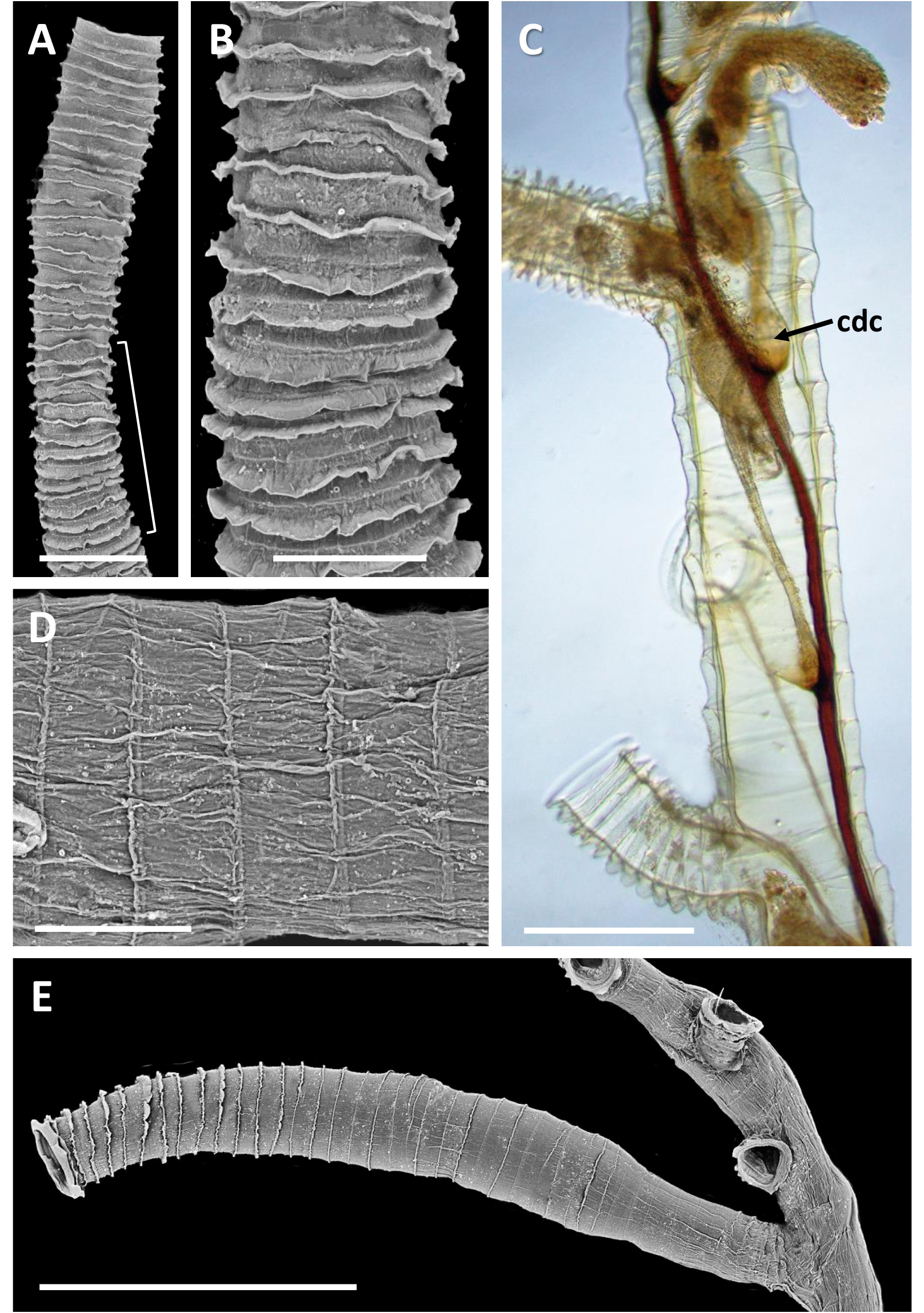
Inception of ringed erect tubes corresponds to a direct mode, except that, inasmuch as principal stems, equivalent to creeping tubes in attached forms, are unattached to a substratum, erect ringed tubes can emerge in any direction. Principal tubes (stems) circular in cross section ( Figs 2C View FIGURE 2 , 11C‒E View FIGURE 11 , 12C‒E View FIGURE 12 ) [diameter 241‒389 (312) μm, n = 30], with fairly regularly spaced annular fuselli encircling the tubes [ Figs 11C View FIGURE 11 , 12D View FIGURE 12 ; distance between annular fuselli 56‒96 (78) μm, n = 30]; tubes c. 4‒12 μm wider at annular fusellar sutures than between annular sutures. Branching of principal tubes initially almost at right angles, between 80° and 90°. No tube fusions or anastomoses. Principal tubes lacking flanking basal layer owing to being unattached; zigzag fusellar sutures lacking.
Erect ringed tubes of fully formed mature feeding zooids ( Fig. 11C, D View FIGURE 11 ) c. 2 mm long, delicate, generally emerging at angles of 70‒80°. Spacing between erect-tube originations relatively close [309‒521 (394) μm, n = 17]. Diameter of erect tubes between fusellar collars narrower [133‒230 (178) μm, n = 50] than principal tubes, with well-developed fusellar collars over much of their length ( Fig. 12A, B View FIGURE 12 ), a majority of collars curled and sinuous in the proximal half to two-thirds of long tubes, as are most of the collars in short tubes. Fusellus height 21‒46 (31) μm ( n = 50), with 15‒21 (18) collars [ n = 25] contained within 500-μm lengths of tube in middle two-thirds of tube length. Fusellar collars weakly projecting 2‒17 (7) μm circumferentially around tube.
Dark, sclerotized tubes 2.2‒3.0 mm long ( Fig. 12E View FIGURE 12 ) occurring sporadically throughout colony, branching off principal tubes to which they are connected internally by a black-stolon branch. As seen by light microscopy, sclerotized tubes darkest proximally, almost black, becoming a little paler distalwards, with the terminal part of the tube transparent. Sclerotized tubes 153‒307 (250) μm diameter (n = 26), tending to be a little narrower proximally and distally, with well-developed fusellar collars in distal half to third of tubes (fusellus height 30‒82 (52) μm; n = 50). Darkened zooids partly visible in transmitted light (bright-field) within sclerotized part of tube; terminal aperture open.
Pectocaulus (black stolon) of principal tubes dark brown in transmitted light, 22‒42 (34) μm diameter [n = 10]. Stolon can run along one side of principal tube for some distance on side opposite erect-tube openings if these open in a series along one side, but can also trace a sinuous course within principal tube where erect tubes open all around ( Fig. 12C View FIGURE 12 ). Stolon bifurcates at angles of c. 35°, sending a branch into another principal tube or sclerotized tube. Transverse septa cross width of principal tubes at angles of c. 30‒35° to compartmentalize zooids. A bowl-shaped or conical diaphragm complex (cdc; Figs 11C View FIGURE 11 , 12C View FIGURE 12 ) accommodates proximal end of each zooidal gymnocaulus. Zooid buds and immature zooids can occur in lineal series within principal tubes prior to lengthening and stretching into developing erect tubes once erect-tube openings established.
Remarks. Rhabdopleura emancipata n. sp. is globally unique among extant rhabdopleurids, distinguished by its loose, tangled, free-living habit. It almost certainly would have originated from an attached base, having been captured by a Menzies Trawl at 318 m depth, but the extent of that attachment is presently unknown. A New Zealand ctenostomatous bryozoan may provide an analogue. Parachnoidea rowdeni Gordon, 2013 begins life as a linearly ramifying encrusting growth on a vent-faunal mussel species that occurs on the Kermadec Ridge. Once established, it produces erect branches that eventually form a much-larger tangle above the mussel. This tangle looks remarkably like that of R. emancipata at the macro level, with free branches of zooids, now somewhat cylindrical, with erect peristomes. The zooids have typical bryozoan anatomy, however. It is not known what induces this morphology in the ctenostome; Gordon (2013) hypothesized that erect growth into free space may have been an adaptation to limited substratum space after initial colonization of the mussel shell, with enhanced access to particulate food, as well as, in this particular setting, compensating for the rate of coating of zooids by metal-sulfide precipitates— growth into three-dimensional space potentially exploits micro-variations in precipitation. There is no evidence of volcanism at the locality where R. emancipata occurred, however.
Could R. emancipata be a free-living form of R. annulata , the type locality of which is c. 460 km distant? Just as free zooids of the ctenostome P. rowdeni are more elongate-cylindrical and stoloniform, could growth into free space induce creeping rhabdopleurid tubes to become cylindrical, thereby modifying the behavior of zooids and suppressing the formation of a zigzag suture in favor of annular sutures? There is considerable overlap in zooid-tube (‘erect’-tube) diameter in R. emancipata and R. annulata [respectively 133‒230 (178) μm vs 167‒200 (180) μm] with practically identical means, but the number of fusellar collars in 500 μm of erect-tube length is non-overlapping (respectively 15‒21 and 11‒13). There is also no equivalent in R. annulata of the blind-ending dark sclerotized tubes in R. emancipata .
The colony of R. emancipata has numerous principal tubes (stems). The distal end of each has a lineal series of zooids developing consecutively distalwards ( Fig. 11E View FIGURE 11 ). Each zooid in turn becomes cut off by a septum and appears to initiate an erect ringed tube via a secondarily formed opening (resorption foramen).
| NIWA |
National Institute of Water and Atmospheric Research |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Graptolithina |
|
Order |
|
|
Family |
|
|
Genus |