Plakortis deweerdtaephila, Vicente, Jan, Zea, Sven & Hill, Russell T., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4178.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:7A957617-C37C-41C8-9A8C-D7BB9178638C |
|
DOI |
https://doi.org/10.5281/zenodo.5617740 |
|
persistent identifier |
https://treatment.plazi.org/id/E622879F-FFD8-CC40-15D9-FDB2FAB80B16 |
|
treatment provided by |
Plazi |
|
scientific name |
Plakortis deweerdtaephila |
| status |
sp. nov. |
Plakortis deweerdtaephila View in CoL sp. nov.
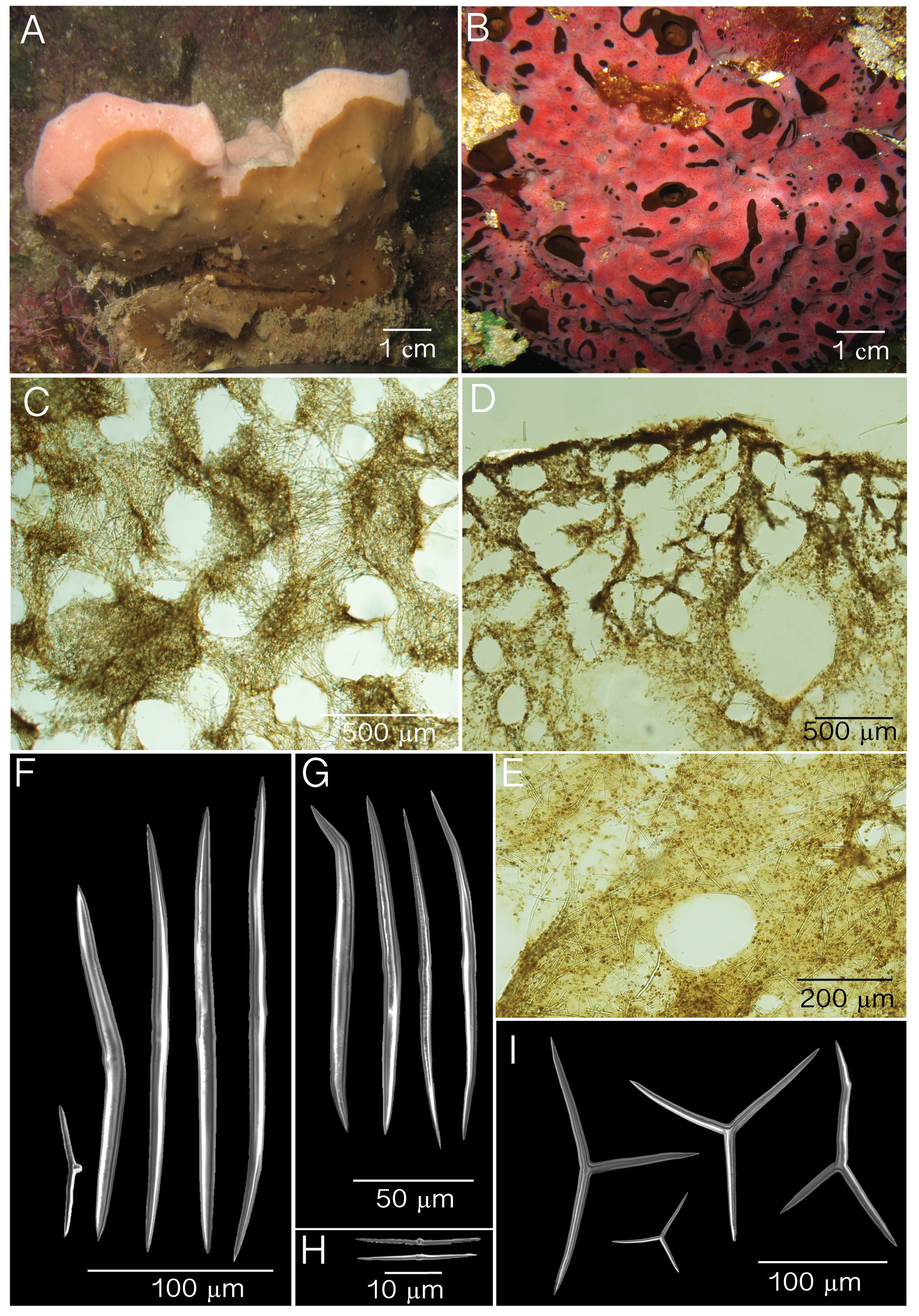
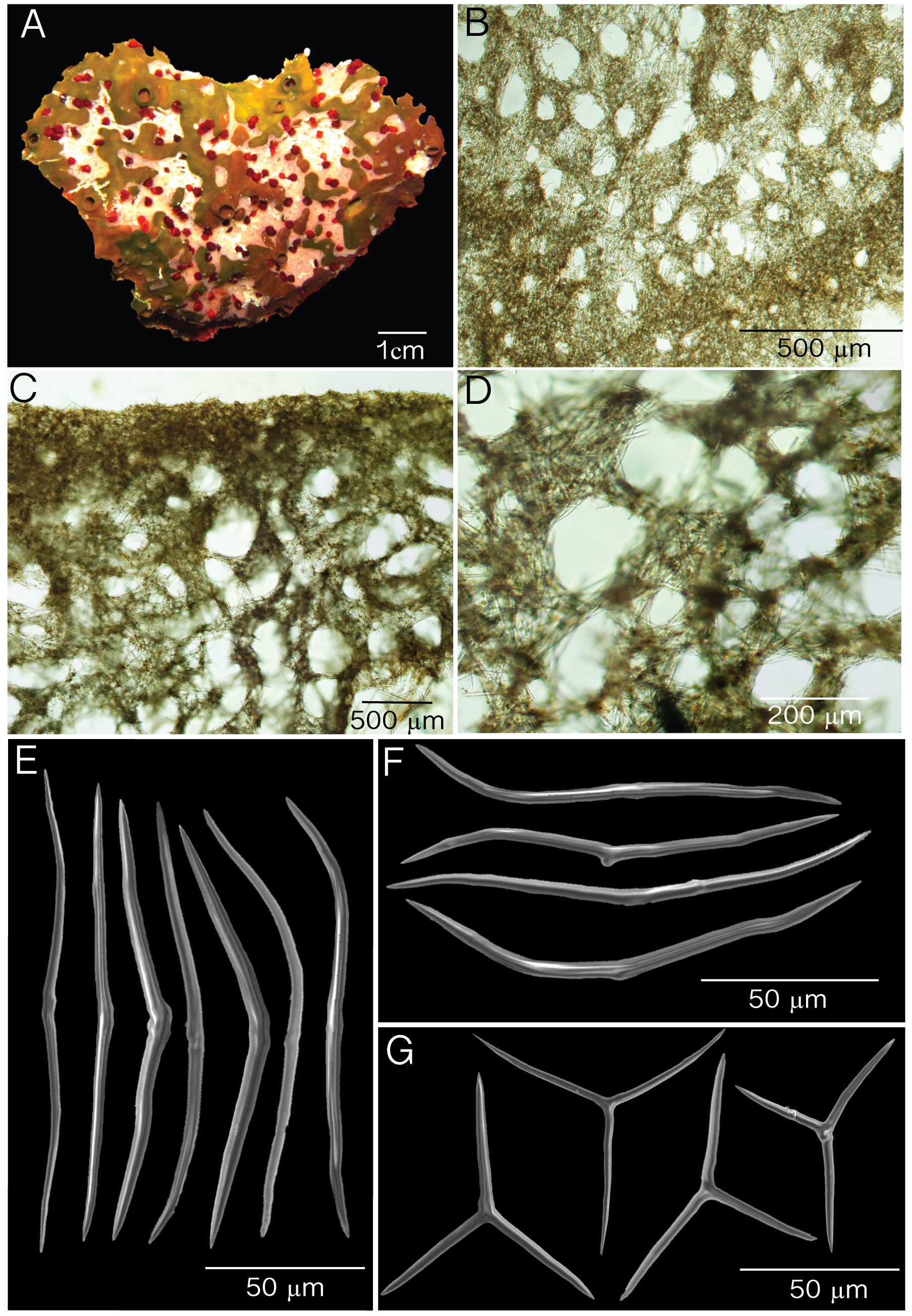
( Fig. 2 View FIGURE 2 ; Table 1)
Plakortis halichondrioides ; Zea et al. 2009 (photographic guide).
Non: Plakortis halichondrioides ( Wilson 1902) , a valid species.
Plakortis sp. 1; Vicente et al. 2014 (ecology and symbiosis).
Plakortis sp. 1-“under Xestospongia deweerdtae associated”; Zea et al. 2014 (photographic guide).
Type material. Holotype and type locality: USNM 12 About USNM 54645, Dolphin Rock , Bocas del Toro, Panama (9.35076° N, - 82.1863° W), 14 m depth, coll. Jan Vicente, May 20, 2015 GoogleMaps . Paratype: USNM 1254647 About USNM , San Salvador, Bahamas (24.0406 ° N, - 74.5314° W), 32 m depth, coll. Jan Vicente, July 19, 2011 GoogleMaps .
Specimens examined for comparison (other than those described here). Plakortis halichondrioides : PHBH, San Salvador (24.0406 ° N, - 74.5314° W), Bahamas, 32 m coll. Jan Vicente, July 19, 2011 GoogleMaps ; PHPR, La Parguera, Puerto Rico (17.8883° N, - 66.9981° W), 32 m depth coll. Jan Vicente, June 12, 2012 GoogleMaps .
Diagnosis. Thinly and thickly encrusting to massive cushions with a soft surface and compressible body. Found always associated as basibiont of X. deweerdtae . The latter may partially cover ( Bahamas) or may completely overgrow P. deweerdtaephila sp. nov. ( Panama). Oscules can be large and slightly elevated ( Bahamas) or can be small and even with the surface when growing underneath X. deweerdtae ( Panama) . Color is dark brown with occasional olive green patches in vivo and exudes a light brown pigment when preserved in ethanol. Reticulated tangential ectosomal skeleton and a vaguely reticulated choanosomal skeleton with lacunae. Spicules are triods, diods and very small diods.
Description. External morphology is influenced by the growth progression of X. deweerdtae on the body of Plakortis deweerdtaephila sp. nov. For example, in Panama it can be thinly encrusting ( Fig. 2 View FIGURE 2 A) growing underneath a thick (1 cm) mat of X. deweerdtae ; very small oscules (1–3 mm). In the Bahamas, sponge pairs form 3 × 30 cm by 1–8 cm thick compressible cushions. X. deweerdtae may overgrow the entire P. deweerdtaephila individual except around the elevated oscules, which are 0.2–0.9 cm in diameter ( Fig. 2 View FIGURE 2 B). Oscules in preserved specimens are contracted. External color is dark brown and internal color is light brown. Surface is smooth, soft and irregular. Consistency is compressible, and easily torn.
Skeleton. Ectosome is composed of a disorganized tangential reticulation of diods and triods. Multispicular tracts are not well defined but form circular meshes, 114– 205 –329 µm diameter (n=20; Fig. 2 View FIGURE 2 C). Spicules never break the surface of the ectosome. When X. deweerdtae forms inner channels within the choanosome of P. deweerdtaephila sp. nov. the ectosome forms a barrier between the two sponge species, as observed in Vicente et al. (2014, their Fig. 7 View FIGURE 7 B). The ectosome (30–50 µm thick) can be easily distinguished from the choanosome with an abundance of subectosomal lacunae and by having a denser aggregation of pigmented cells ( Fig. 2 View FIGURE 2 D). The choanosome is dense with a confused reticulation of diods and triods that form circular meshes of varying diameters ( Fig. 2 View FIGURE 2 E).
Spicules. Diods can be very small to large. Large diods are slightly bent but mostly straight, slightly sinuous, with a thick center. Ends of large diods are sharp and sometimes bent ( Fig. 2 View FIGURE 2 F–G): Small diods are rare, also thicker in the center and mostly straight. Small diods in Panama ( Fig. 2 View FIGURE 2 F) can be as small as 50 µm and in the Bahamas ( Fig. 2 View FIGURE 2 H) can be as small as 24 µm. Size (length × width) for Panama, 50– 173.2 (±37.1)–234 µm × 4.3– 7.9 (±1.7)– 11.0 µm; Bahamas, 24– 107.6 (±43.4)–172 µm x 2.4– 3.7 (±0.7)–4.8 µm ( Table 1). Triods are not abundant, being Yshaped, smooth, and with sharp endings that are sometimes bent ( Fig. 2 View FIGURE 2 I) Size for Panama, 40– 64.2 (±15.8)–103 µm × 1.8– 5.9 (±2.2)–10.9 µm; Bahamas, 26– 45.1 (±11.3)–67 µm × 2.4– 3.3 (±0.5)–4.8 ( Table 1). Microrhabds, quasiamphiasters and spheres are absent.
Spicule Specimen Location* Length (µm) Width (µm)
Diod Plakortis deweerdtaephila USNM1254645 (h) BDT, Panama 50– 173.2 (±37.1)–234 4– 7.9 (±1.7)–11 Plakortis deweerdtaephila USNM1254647 (p) SS, Bahamas 24– 107.6 (±43.4)–172 2– 3.7 (±0.7)–5 Plakortis symbiotica USNM1254650 (h) LP, Puerto Rico 72– 113.1 (±16.7)–142 2– 3.6 (±0.8)–5 Triods Plakortis deweerdtaephila USNM1254645 (h) BDT, Panama 40– 64.2 (±15.8)–103 1– 5.9 (±2.2)–11 (actine) Plakortis deweerdtaephila USNM1254647 (p) SS, Bahamas 26– 45.1 (±11.3)–68 2– 3.3 (±0.5)–5 Plakortis symbiotica USNM1254650 (h) LP, Puerto Rico 20– 40.4 (±12.8)–71 2– 3.3 (±0.7)–5
*Location BDT refers to Bocas del Toro, SS refers to San Salvador and LP to La Parguera.
Habitat and ecology. Extensive surveys performed in the Caribbean suggest that this sponge is obligately associated with X. deweerdtae as free-living forms of P. deweerdtaephila sp. nov. have not been observed in more than 25 surveys that spanned four countries in the Caribbean: Mexico, Bahamas, Puerto Rico ( Vicente et al. 2014) and Panama in this study. P. deweerdtaephila / X.deweerdtae sponge pairs have been documented from small sponge recruits to massive adults ( Fig. 3 View FIGURE 3 A–C in Vicente et al. 2014). These sponge pairs are found on the upper level (30– 36 m) of mesophotic reef habitats, on vertical walls, shaded sides of pinnacles, as well as cryptic habitats (roof of overhangs and reef caves) of the Caribbean. In Panama the new species was found in a depth of 14 m, on the shaded sides of spur and groove hard bottom habitats exposed to high wave energy; individuals are completely overgrown by X. deweerdtae to the point where the new species is not visible unless the sponge pair is broken.
Distribution. Bahamas (Little San Salvador, San Salvador, Acklins, Mayaguana, Mira Por Voz, Plana Keys, Hogsty Reef; see also Vicente et al. 2014, Zea et al. 2014) and Panama (Dolphin Rock, Bocas del Toro) ( Fig. 1 View FIGURE 1 B, E).
Etymology. The name deweerdtaephila denotes the close association with Xestospongia deweerdtae , from phila meaning “living or growing by preference”.
Taxonomic remarks. There are currently 12 Plakortis species known for the TWA of which seven occur in the Caribbean: P. angulospiculatus ( Carter 1883) , P. zyggompha ( De Laubenfels 1934) , P. halichondrioides ( Wilson 1902) , P. myrae Ereskovsky et al., 2014 , P. e d w a rd s i Ereskovsky et al., 2014, and P. da r i a e Ereskovsky et al., 2014. P. deweerdtaephila sp. nov. does not have any microrhabds or quasiamphiasters which places it among the P. simplex species group according to Muricy (2011). This species complex in the TWA includes, P. insularis ( Moraes & Muricy, 2003) , P. zyggompha , P. edwardsi , and P. dariae . By having two size classes of diods the new species is more similar to P. e dw ard s i and P. dariae that inhabit vertical shaded sides of reef boulders in Martinique and were collected along depths of 22– 26 m.
The external morphology of the new species differs from P. e d w a rds i by having an irregular surface with oscules that can be elevated with large openings. In P. e d w a rds i oscules are flush with the surface ( Ereskovsky et al. 2014). The ectosome of the new species has more organized circular meshes than P. edwardsi , without spicules cluttering open circular spaces. There is also an abundance of subectosomal lacunae that separates the ectosome from the choanosome in the new species that is not mentioned in the description of P. e d w a rd s i. The choanosome also has more abundant circular meshes than in P. e d w a rds i. Spicules of specimens from the Bahamas, however, are similar in size and shape to P. e dw ard s i.
Despite having similar spicule sizes to P. dariae , the new species differs in external morphology and in the skeleton arrangement of the ectosome and choanosome. Also, the color of P. dariae is green, contrasting with the brown of the new species. Sponge individuals of the new species are larger and thicker than P. dariae . The new species also has larger oscules and an irregular surface. The ectosome of P. dariae is poorly differentiated without subectosomal lacunae. Spicules of P. d ar i ae cross the surface of the ectosome while spicules in the ectosome of the new species never cross the surface. The shape of the small diods of P. d ar i ae is also irregular with one end blunt which does not coincide with the shape of small diods in the new species that are always symmetrical.
The specimen also shows morphological differences to P. insularis , in that it has an irregular surface with large oscules. The skeleton of P. insularis consists of a loose and confused arrangement of diods in low density and in P. deweerdtaephila diods are present in high density forming organized circular meshes. The dense arrangement of diods also forms subectosomal lacunae which are not present in P. insularis . Small diods in P. insularis are also absent.
The thick massive shape, brown color and dark color exudate, plus having some spicules reaching sizes>150 µm, led Zea et al. (2009) to erroneously identify P. deweerdtaephila as P. halichondrioides . This fact was later corrected (Zea et al. 2014) after the molecular and spicular comparisons of Vicente et al. (2014) demonstrated their distinctiveness. To understand their differences, we made direct comparisons of specimens of P. deweerdtaephila with specimens of P. halichondrioides from the Bahamas and Puerto Rico. Morphologically, the ectosomal skeleton of P. halichondrioides can be distinguished from P. deweerdtaephila in that spicules break the surface of the ectosome. Spicules also protrude inside circular meshes of the ectosome of P. halichondrioides and in P. deweerdtaephila circular meshes are free of spicules. Spicule sizes are also significantly smaller in P. deweerdtaephila than in P. halichondrioides ( Vicente et al. 2014) . Differences between P. deweerdtaephila and P. symbiotica are given below in the taxonomic remarks of the latter species.
Recently, P. angulospiculatus was reported from Carrie Bow Cay, Belize, completely overgrown by X. deweerdtae ( Rützler et al. 2014) . Spicule size and shape of the P. angulospiculatus identified by Rützler et al. (2014) are in agreement with P. deweerdtaephila . Thus, its identity needs to be confirmed through molecular analysis, as the sequences of P. angulospiculatus we used herein (see below) turned out to be distantly related to those of P. deweerdtaephila or P. symbiotica . P. angulospiculatus is a widespread Caribbean species, and its many reports may encompass several of the species newly described in the last decade (e.g., Ereskovski et al. 2013; Domingos et al. 2013); it needs to be reassessed, preferably from holotype material. The cob and cox1 gene sequences we used from P. angulospiculatus individuals were determined by Ereskovski et al. (2014) and Erpenbeck et al. (2008), respectively. The only character that apparently may distinguish P. angulospiculatus from our two new species of Plakortis is its lack of dark brown exudate (Ereskovski et al. 2013), but even this needs to be confirmed.
The most important morphological character that differentiates P. deweerdtaephila from any Plakortis species is that individuals have only been found associated with X. deweerdtae . No other sponge associations between Plakortis and a Xestospongia have been reported other than the P. symbiotica / X. deweerdtae sponge pair (described below). This is the first time that an obligate symbiosis with a heterospecific sponge species is a taxonomic character of any sponge species.
The larger size of spicules in specimens from Panama in comparison to those from the Bahamas ( Table 1), reflect a Caribbean wide geographical pattern present in many groups of sponges, attributed to the apparent enrichment of silicon that continental locations experience, in comparison to oceanic ones ( Zea 1987).
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |