Pelodytes ibericus Sánchez-Herráiz, Barbadillo, Machordom, and Sanchiz, 2000
|
publication ID |
https://doi.org/10.11646/zootaxa.4243.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CE502CF7-6F19-43A2-AD79-15DBE777E28B |
|
DOI |
https://doi.org/10.5281/zenodo.6016572 |
|
persistent identifier |
https://treatment.plazi.org/id/038A7153-707E-BC6A-95A2-F913FB8F5E76 |
|
treatment provided by |
Plazi |
|
scientific name |
Pelodytes ibericus Sánchez-Herráiz, Barbadillo, Machordom, and Sanchiz, 2000 |
| status |
|
Pelodytes ibericus Sánchez-Herráiz, Barbadillo, Machordom, and Sanchiz, 2000 View in CoL
Iberian Parsley Frog
( Fig. 10 View FIGURE 10 )
Identity and morphology. This species was described and diagnosed from P. punctatus (including populations that we here consider as P. hespericus sp. nov.) based on morphometric, osteological and genetic (allozyme and mtDNA) differences by Sánchez-Herráiz et al. (2000). Its name-bearing type ( holotype) is MNCN 23662 About MNCN from "Doñana Biological Reserve ( 36 59′ N, 6 27′ W, elevation 10 m), Province of Huelva ( Spain) ( Sánchez-Herráiz et al. 2000; Frost 2015). This locality is well within the range of genetic lineage B (localities 50 and 52 in Díaz- Rodríguez et al. 2015, with specimens genotyped) and therefore assignment of this lineage to the nomen P. ibericus is straightforward. GoogleMaps
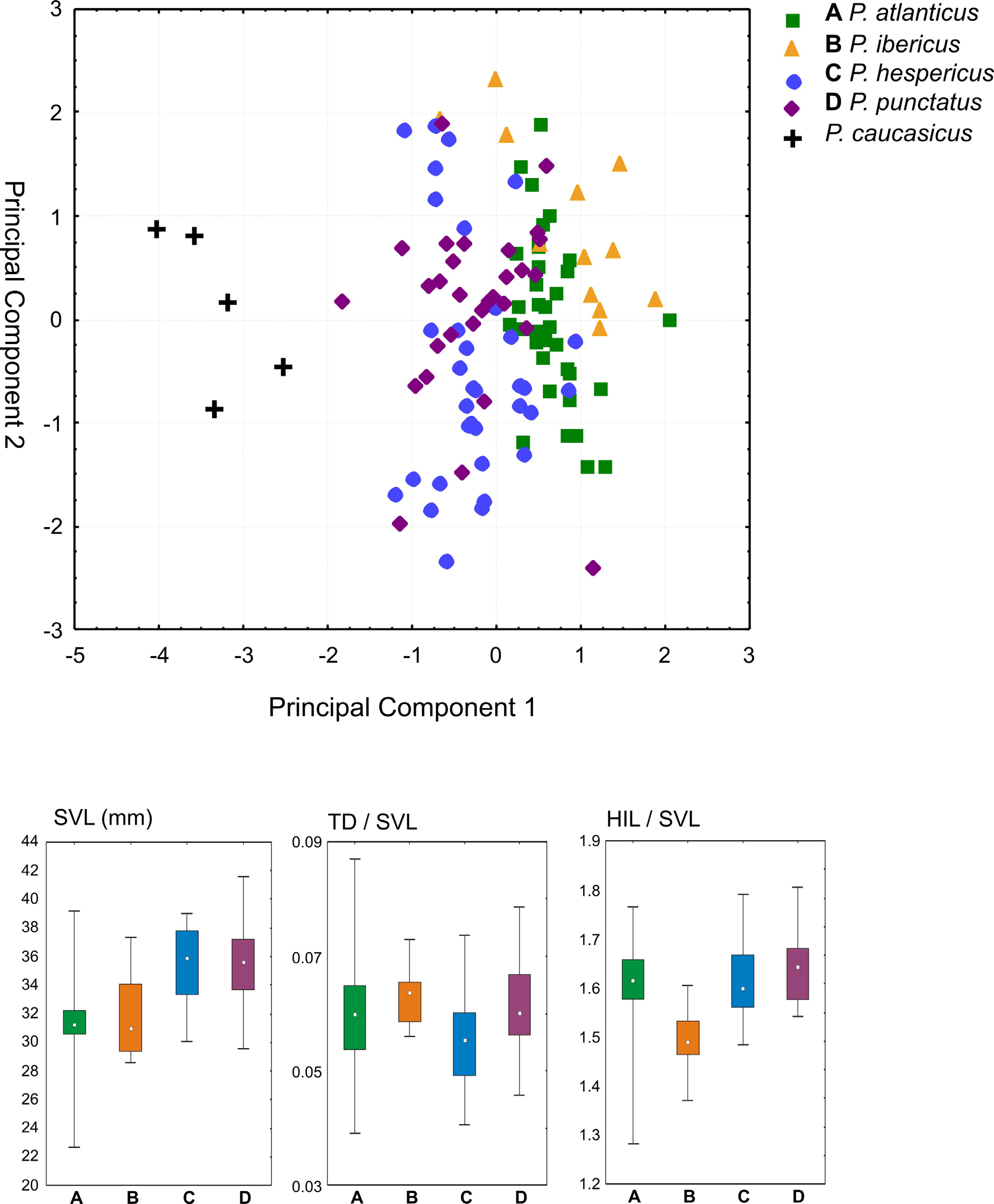
Pelodytes ibericus View in CoL is smaller than P. hespericus View in CoL sp. nov. and P. punctatus View in CoL , with a mean SVL of 31.2 (maximum SVL 37.2 mm) in males, and 33.3 mm ( 42.2 mm) in females (see Table 3 View TABLE 3 ). Average weight is 3.5 g in males and 4.9 g in females. The species is somewhat similar morphologically to P. atlanticus View in CoL sp. nov., but most specimens have shorter limbs ( Fig. 6 View FIGURE 6 ) and an overall more compact body. Numerous differences in osteology have been detected by Sánchez-Herráiz et al. (2000) and Sanchiz et al. (2002), especially in the cranium, including a less elongated tectum nasi, larger scapular crista anterior, shorter parahyoid bone, and frontoparietals medially more widely separated in P. ibericus View in CoL than in P. punctatus View in CoL . The proximal subarticular tubercles of P. ibericus View in CoL have been described as irregular and protruding conically, whereas in P. hespericus View in CoL sp. nov. they are often more rounded (Sánchez- Herráiz et al. 2000) but the variation of this character requires more careful study. Quite often P. ibericus View in CoL has dorsally two crossed pale bands forming an X-shaped pattern.
Distribution. The distribution range comprises a large area in the Southern Iberian Peninsula: from southeastern ( Beja, Faro and Setúbal) to central provinces ( Évora and Portalegre) of Portugal ( Pargana et al. 2003; Crespo et al. 2008; van de Vliet et al. 2012), and in Spain, from northern Extremadura (Badajoz) ( Palomo 1993; Da Silva 1994; Avilés et al. 1999; Muñoz del Viejo et al. 2005) and southern Castilla-La Mancha (Ciudad Real) ( Ayllón et al. 2003; Sánchez-Herráiz 2004; Díaz-Rodríguez et al. 2015) to Andalucía. In this region, where 90% of its global distribution occurs, it is widely spread across the provinces of Huelva and Cádiz but rare in the east ( Busack 1977; Fernández-Cardenete et al. 2000; Ceacero et al. 2007). Recent studies have outlined more precisely the distribution based on molecular markers (van de Vliet et al. 2012; Díaz-Rodríguez et al. 2015). The exact range limits are still partly unknown and new detailed molecular analyses of contact zones with other lineages are ongoing ( Díaz-Rodríguez 2016).
Natural history. Notes on the natural and life history of this species have been provided by Díaz-Paniagua (1983, 1986, 1989, 1990), Tejedo (1991), Avilés et al. (1999), Barbadillo et al. (1999), Sánchez-Herráiz (2004), Díaz-Paniagua et al. (2005), and Reques (2014). Pelodytes ibericus reproduces in ephemeral water bodies, mainly temporary streams and seasonal ponds located around agricultural crops, grassland, scrub and oak from Mediterranean savanna. It tolerates some salinity, even at the larval stage, reproducing sometimes in salty coastal wetlands and inland brackish water lagoons ( Reques & Tejedo 2014). It tolerates a variety of substrates (siliceous, clay and calcareous). Phenological changes occur in dry years ( Díaz-Paniagua 1989; Sánchez-Herráiz 2004). Breeding starts early in the season, avoiding interspecific competition ( Díaz Paniagua 1988), often during autumn rains ( October – November) in southern Portugal and Western Andalusia ( Busack & Jaksic 1982; Díaz-Paniagua & Rivas 1987; González de la Vega 1988; Sánchez-Herráiz 2004; Reques 2014), and sometimes extends to March at higher latitudes ( Badajoz: Avilés et al. 1999) and altitudes ( Sierra de Cabra: Reques & Tejedo 1991; Sierra Morena: Reques 2000). Sexual maturity is reached at an age of one year ( Sánchez-Herráiz 2004) and the maximum age is around six years ( Reques 2014). The species is threatened by fragmentation and destruction of breeding habitat ( van de Vliet et al. 2012), groundwater overexploitation and water pollution ( Salvador & García-París 2001; Barbadillo 2002a; García-Muñoz et al. 2011) and predation by introduced species ( Ceacero et al. 2007; Bosch et al. 2009; Nunes et al. 2014). Its IUCN threat status is Least Concern ( Bosch et al. 2009) but a careful reassessment of its situation might be needed (Franco & Rodríguez de los Santos 2001; Pleguezuelos et al. 2002; Barbadillo 2002b; Reques et al. 2006).
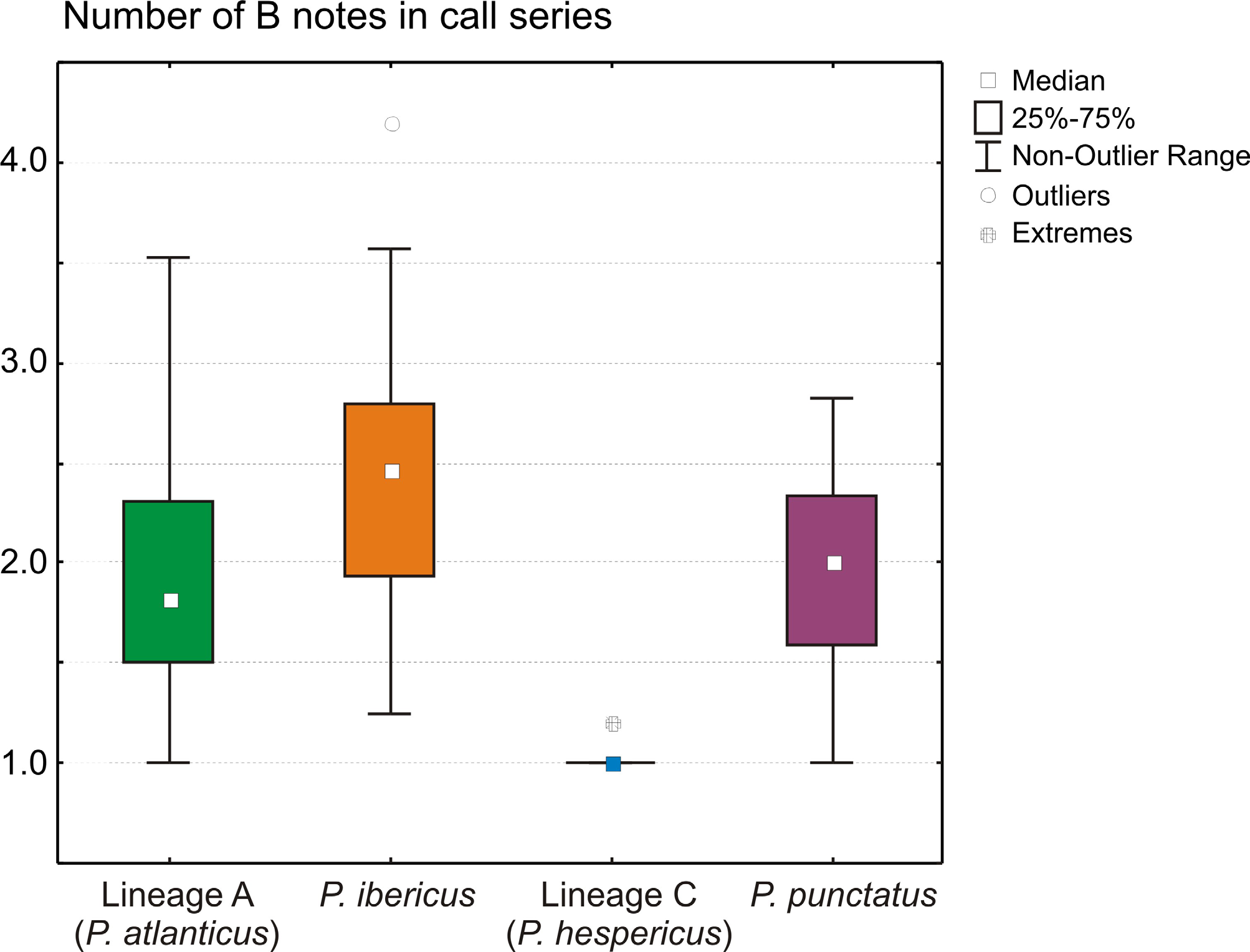
Advertisement call and reproductive behavior. The advertisement call of P. ibericus was studied by Paillette et al. (1992), Márquez et al. (2001), Sánchez-Herráiz (2004), Pargana et al. (2003), and Díaz-Paniagua et al. (2005). It is similar to that of other western Pelodytes lineages, consisting of two notes ( A and B) with typically a series of various B notes following one A ( Fig. 7 View FIGURE 7 ). Each note has pulses distinctly spaced in the beginning and more concentrated towards the end; the pulsed part is relatively longer in the B note (Paillete et al. 1992; Pargana 1998; Sanchez-Herráiz 2004). Calling specimens are in or near the water, but they do not emit their vocalizations fully submerged. Reproductive activity takes place before and after sunset, mostly during rainy days accompanied by slight increases of environmental temperature, although they can also exhibit diurnal activity on rainy days ( Avilés et al. 1999; Barbadillo et al. 1999).
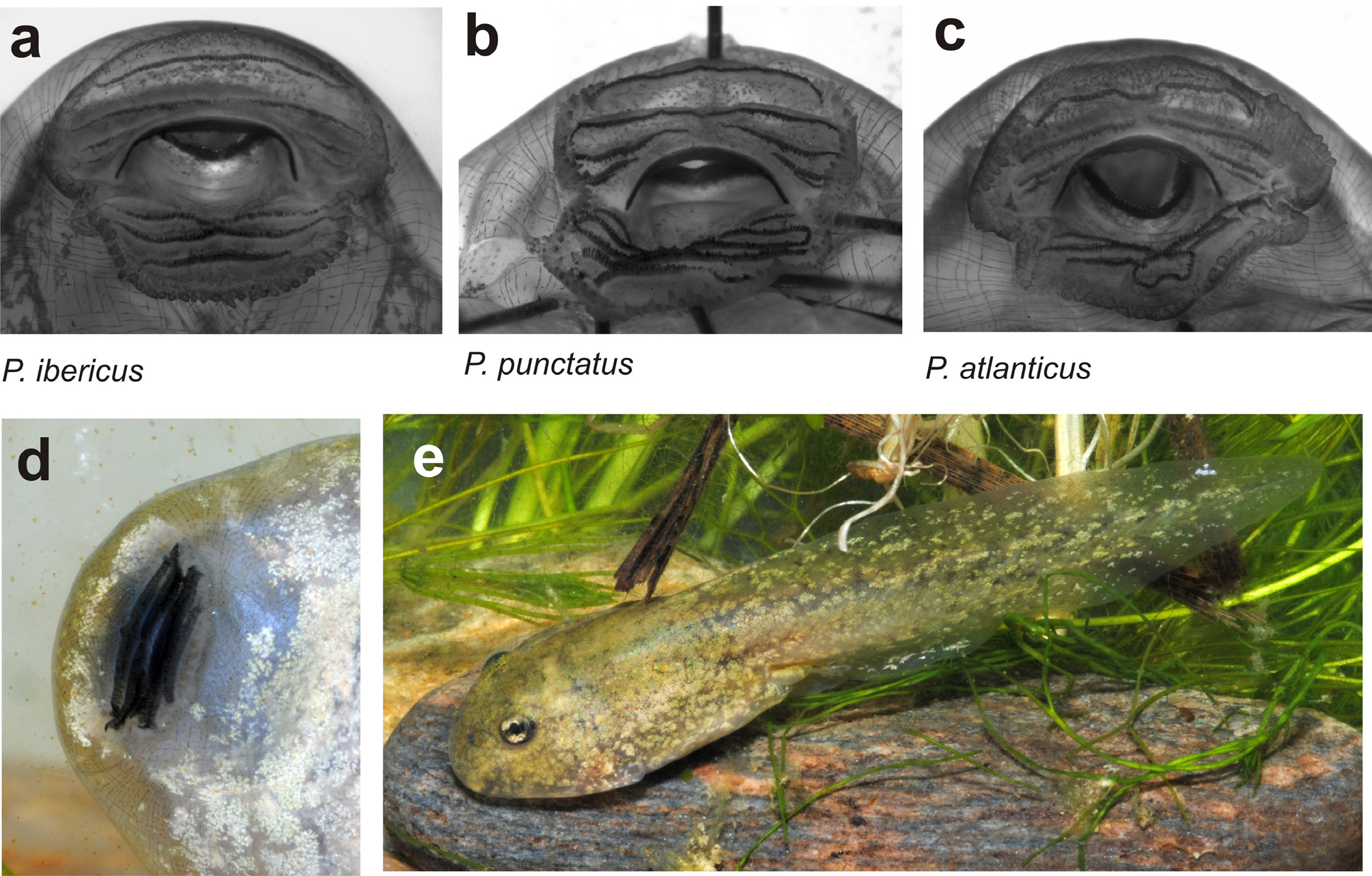
Tadpole. We assessed morphological characters in one tadpole in developmental stage 36 (field number ZCMV 14041, collected at Grazalema, Cádiz, BL 25.5 mm, TL 48.7 mm). The external morphology of this tadpole and two other specimens has a close similarity to those of P. punctatus except that the upper third row of keratodont has a very tiny gap only or it is even absent, giving LTRF 4(2–4)/5(1–3) or 4(3–4)/5(1–3) and 5(3–5)/5(1–3) or 5(3–5)/5(1–3) ( Fig. 9a View FIGURE 9 ).
Larvae have an omnivorous diet composed of algae, mainly Euchlorophyta and Chrysophyta, aquatic plants, fungi, arthropods and small debris ( Díaz-Paniagua 1989; Díaz-Paniagua et al. 2005). Tadpoles of P. ibericus also predate upon embryos of other anurans (such as Epidalea calamita ; Tejedo 1991).
| MNCN |
Museo Nacional de Ciencias Naturales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.