Paraustrosimulium anthracinum (Bigot)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4337.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:BA7E7DE5-25C2-41BA-8642-9B429FDC5294 |
|
DOI |
https://doi.org/10.5281/zenodo.6052670 |
|
persistent identifier |
https://treatment.plazi.org/id/9D4187CA-FFEA-FFCD-ADF6-55DCFF3FB031 |
|
treatment provided by |
Plazi |
|
scientific name |
Paraustrosimulium anthracinum (Bigot) |
| status |
s.str. |
Paraustrosimulium anthracinum (Bigot) View in CoL
( Figs. 86–128 View FIGURES 86 – 91 View FIGURES 92 – 96 View FIGURES 97 – 101 View FIGURES 103 – 106 View FIGURES 107 – 110 View FIGURES 111 – 115 View FIGURES 116, 117 View FIGURES 121 – 126 View FIGURES 127, 128 )
Simulium anthracinum Bigot, 1888: 15 View in CoL .
Simulium anthracinum View in CoL . Kertész 1902: 285.
Simulium (Austrosimulium) anthracinum View in CoL . Edwards 1931: 143.
Simulium (Austrosimulium) anthracinum View in CoL . Pinto 1931: 671.
Austrosimulium anthracinum . Smart 1945: 499. Vargas 1945: 113. Wygodzinsky 1953: 293. Dumbleton 1960: 543. Austrosimulium (Paraustrosimulium) anthracinum . Wygodzinsky & Coscarón 1962; 242. Coscarón 1968: 66. Paraustrosimulium anthracinum View in CoL . Crosskey 1969: 73. Crosskey & Howard 1997: 18; 2004: 10. Prosimuliini View in CoL . Paraustrosimulium anthracinum View in CoL . Adler & Crosskey 2008: 26. Transferred to Simuliini View in CoL . Paraustrosimulium anthracinum View in CoL . Adler & Crosskey 2017: 30.
Paraustrosimulium anthracinum View in CoL . Hernández-Triana et al. 2017: 350.
Simulium moorei Silva Figueroa, 1917: 30 .
Simulium (Austrosimulium) moorei . Edwards 1931: 144. Simulium moorei . Pinto 1931: 29.
Simulium moorei . Vargas 1945: 166.
Austrosimulium moorei . Smart 1945: 499.
Austrosimulium moorei . Wygodzinsky 1953: 298. Synonymized with A. anthracinum View in CoL .
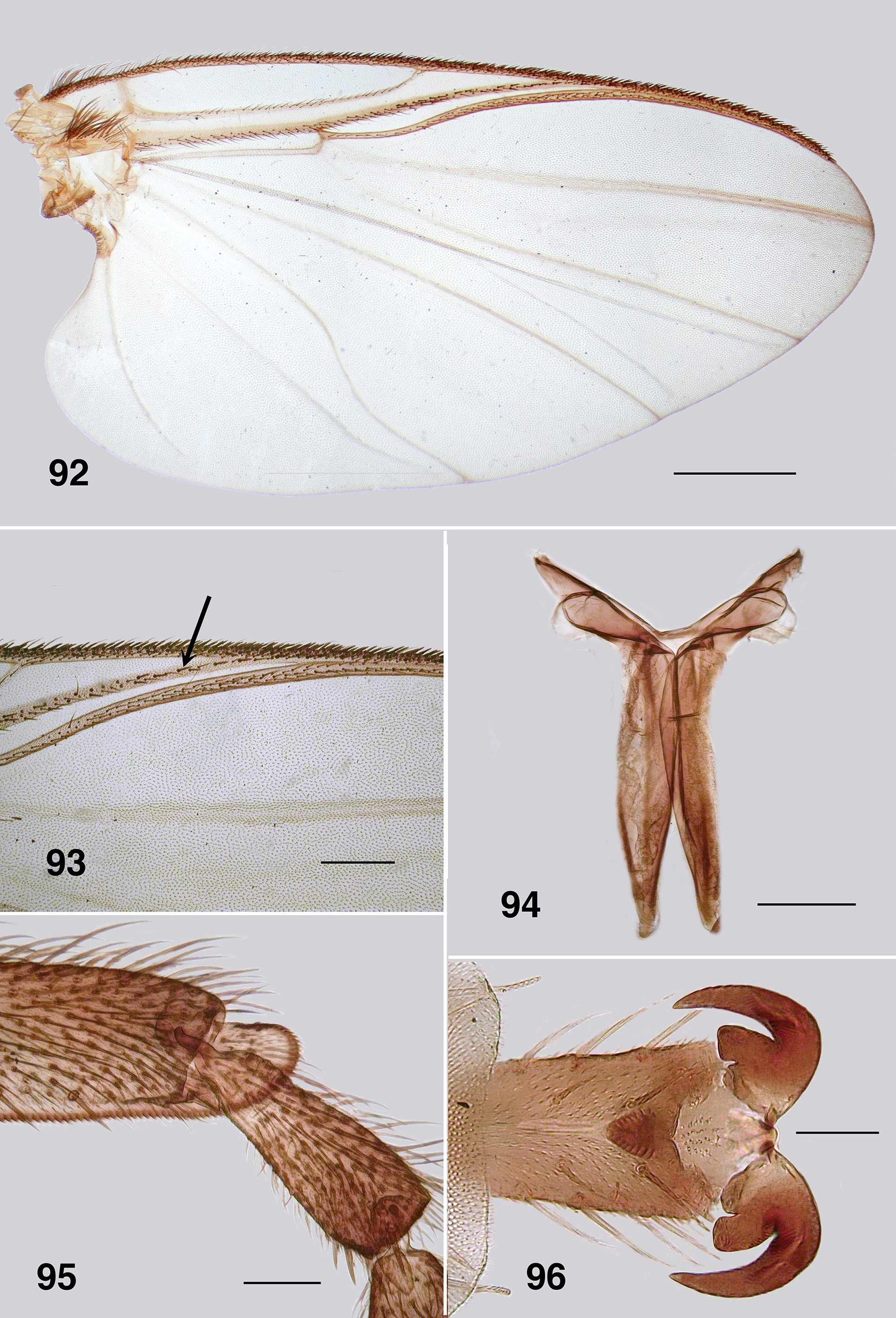
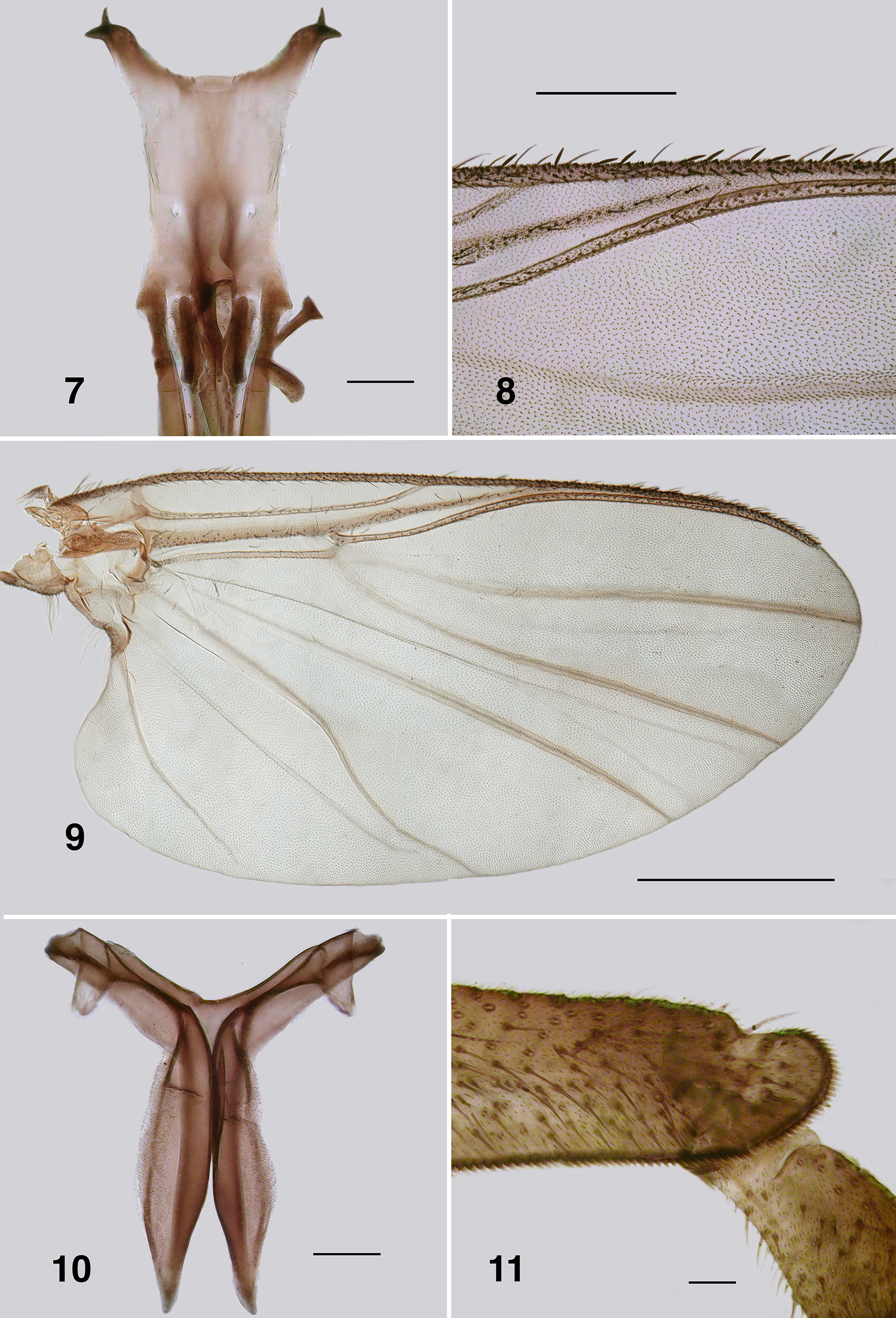
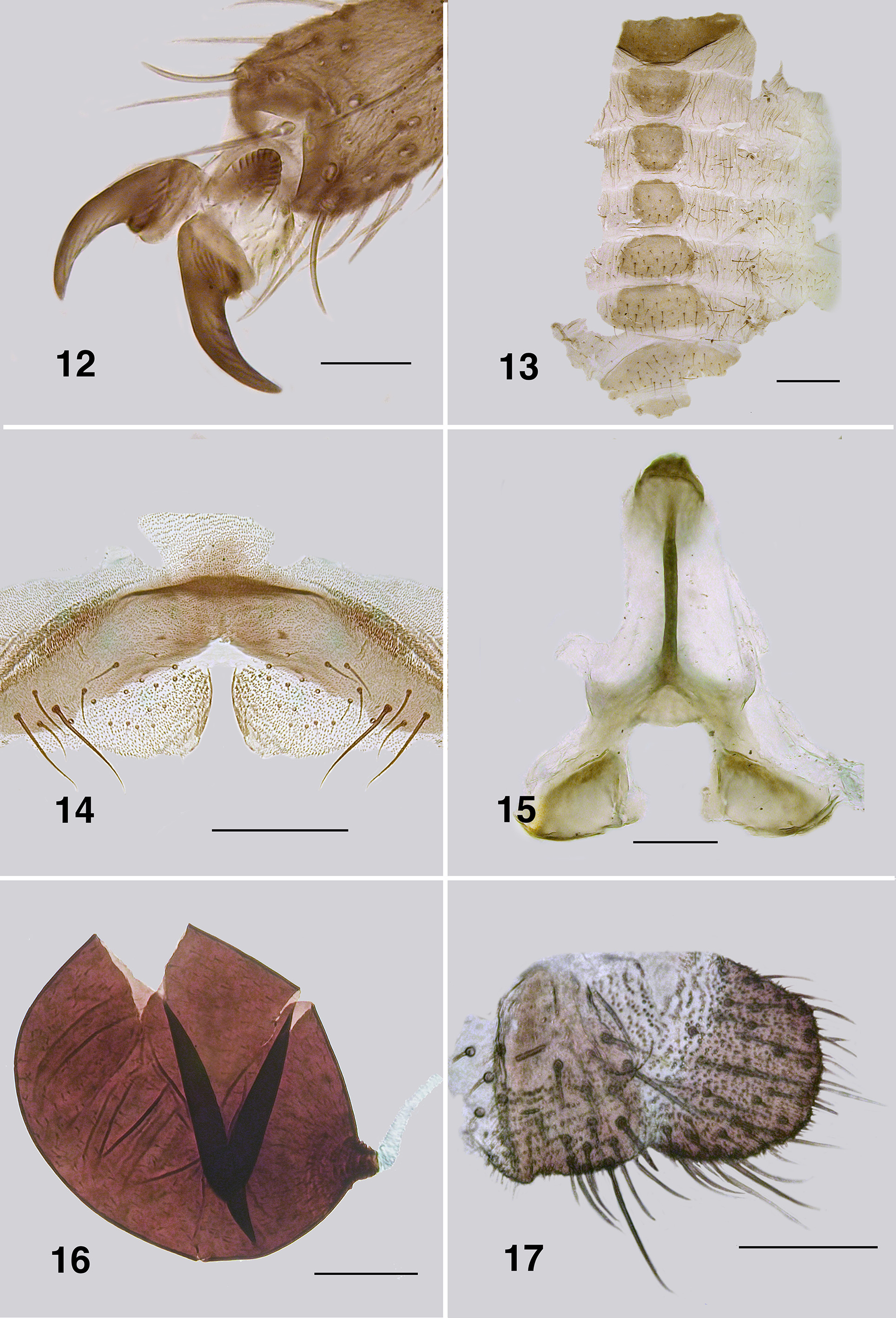
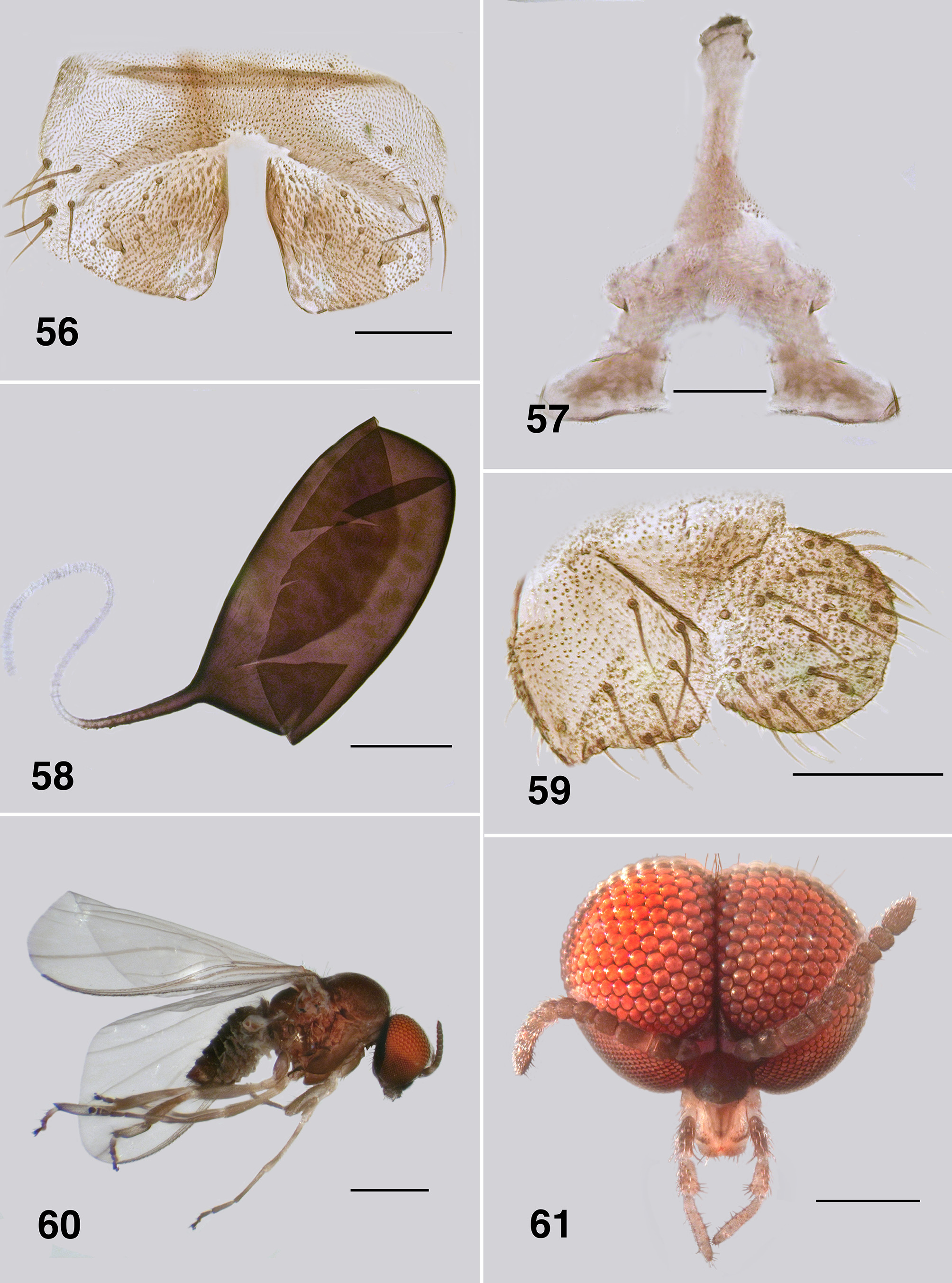
Redescription. Adult female (based on 5 specimen and literature description). Body ( Fig. 86 View FIGURES 86 – 91 ): general body colour in alcohol evenly dark brown; total length 3.3 mm. Head ( Fig. 87 View FIGURES 86 – 91 ): width 0.76 mm; depth 0.49 mm; postocciput black, markedly hirsute; frons dark brown; frons–head ratio (narrowest width of frons: greatest width of head) 1.0:7.0. Eyes: evenly reddish brown, interocular distance 0.11 mm; ommatidia 0.012 mm in diameter; ca. 36 rows up and across at mid–eye. Clypeus: light brown; 0.23 mm wide; vestiture of sparse pale hairs. Antenna ( Fig. 88 View FIGURES 86 – 91 ): total length 0.68 mm; 8 flagellomeres, pedicel and scape small, wider than long, distal region of scape pale, remainder of antenna brown; flagellomeres quadratic, overall slightly tapered, apical flagellomere longer than broad. Mouthparts: well expressed, ca. 0.5 length of head depth; maxillary palpus ( Fig. 89 View FIGURES 86 – 91 ), total length 0.6 mm, articles brownish black, 3rd article black; proportional length of 3rd, 4th and 5th articles 1.0:0.8:1.2; sensory vesicle spherical, 0.5x width of 3rd article, opening 0.3x width of vesicle; mandible ( Fig. 90 View FIGURES 86 – 91 ), not markedly expanded apically, 27 inner teeth and 10–12 finely pointed outer teeth; lacinia ( Fig. 90 View FIGURES 86 – 91 ) with 11 inner teeth and 13 outer teeth; cibarial cornuae ( Fig. 91 View FIGURES 86 – 91 ) with marked sculptured edges, central depression broad and shallow. Lateral cervicales: well developed ( Fig. 86 View FIGURES 86 – 91 ). Thorax: length 1.5 mm; width 1.0– 1.1 mm; scutum evenly dark brown, vestiture of evenly distributed recumbent silver hairs; postpronotal lobe with longer hairs; antepronotal lobe with markedly longer dense hairs; scutellum pale, and postnotum lighter than scutum; scutellar depression and scutellum with pale hairs; pleuron and anepisternal membrane dark brown, bare. Wing ( Fig. 92, 93 View FIGURES 92 – 96 ): length 3.0– 3.1 mm; width 1.6 mm, anterior veins lightly pigmented, basal cell distinct, costa not extended to wing apex with spiniform setae distally, also present on R1 vein; radial veins not markedly closely applied to costa; a:b ratio 1.0:2.8; M thickened apically; A2 not approaching wing margin. Haltere: dark grey. Metathoracic furcasternum: dorsal arms with rounded projections ( Fig. 94 View FIGURES 92 – 96 ). Legs: overall dark brown; hind basitarsus ca. 5.7x as long as its greatest breadth, very slightly expanded medially, ventral row of stout spines present, calcipala moderately expressed, pedisulcus not markedly developed ( Fig. 95 View FIGURES 92 – 96 ); tarsal claw ( Fig. 96 View FIGURES 92 – 96 ) with moderately developed basal heel and markedly angulate tooth and notch. Abdomen ( Fig. 97 View FIGURES 97 – 101 ): abdominal scale black with dark hairs, not greatly extended; tergite II 3.2x wider than long, shallowly V–shaped, sparse black hairs, III, IV essentially bare, III–V as wide as long with rounded corners, VI 2x wider than long; dorsal vestiture of small sparse black hairs increased in density posteriorly. Genitalia: sternite VIII vestiture of sparse coarse black hairs posterolaterally; hypogynial valves ( Fig. 98 View FIGURES 97 – 101 ) broadly rounded; median edges slightly convex, not strengthened along edge, bluntly rounded apically, slightly crenulated laterally; genital fork ( Fig. 99 View FIGURES 97 – 101 ) with anterior arm broad (not easily observed), slightly sclerotized and pigmented medially (not so according to Wygodzinsky & Coscarón 1973), more so anteriorly, lateral arms broad, indications of lateral apodeme (as in Gigantodax ), apodeme (as in Austrosimulium ) present only as ridge, lateral arms broad, lateral plates large, pointed posteromedially, angulate posterolaterally; spermatheca ovoid ( Fig. 100 View FIGURES 97 – 101 ), length 0.14 mm, surface un–patterned; sparse internal acanthae; no clear area at junction of spermathecal duct, pigmentation not extended down duct; cercus ( Fig. 101 View FIGURES 97 – 101 ) broadly rounded apically, sloped ventrally, vestiture of evenly spaced hairs, anal lobe not angulate posteriorly.
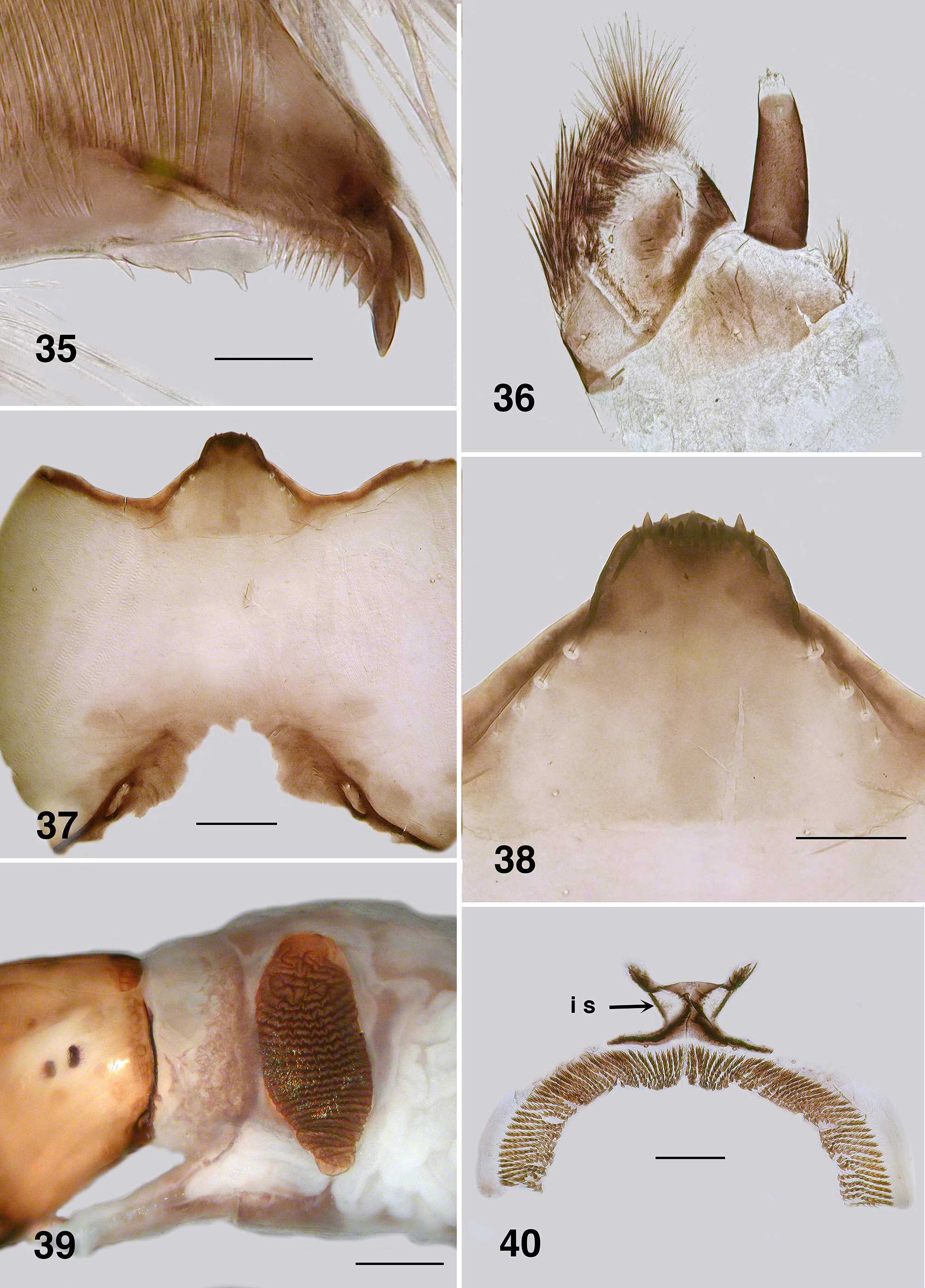
Adult male (reared and other specimens). Body (Fig. 102): general colour dark brown to black; total length 2.3– 2.6 mm. Head ( Fig. 103 View FIGURES 103 – 106 ): width 0.90 mm; depth 0.62 mm. Eyes: upper ommatidia very dark red, 0.03 mm in diameter, ca. 22 across, 20 down; lower ommatidia almost black, 0.01 mm in diameter, ca. 35 across, 26 down. Clypeus: blackish brown; vestiture of very sparse black hairs; 0.2 mm wide. Antenna ( Fig. 104 View FIGURES 103 – 106 ): total length 0.75 mm; scape and pedicel black, first flagellomere and remainder dark brown; pedicel longer and wider than other divisions; first flagellomere longer than wide, others subrectangular; non–tapered, narrow in comparison to that of female; apical article ca. 2x longer than wide. Mouthparts: moderately developed; 0.5x head depth; mandibles insubstantial, finely tapered with apical hairs; laciniae finely tapered apically with terminal hairs; maxillary palpus ( Fig. 105 View FIGURES 103 – 106 ), dark brown, 0.55 mm long, proportional lengths of 3rd, 4th and 5th articles 1.0:0.8:1.4, sensory vesicle small, occupying 0.25x width of article, opening 0.5x width of vesicle. Cervical sclerites well developed. Thorax: length 1.5 mm; width 0.9 mm; in alcohol, scutum evenly blackish brown, vestiture of fine recumbent pale hairs (often worn); scutellum and postscutellum concolourous with scutum. Wing: 3.0 mm in length, 1.5 mm in width; otherwise as for female. Haltere: tan. Legs: yellowish blackish brown; hirsute; hind basitarsus ca. 4.5x as long as its greatest breadth, slightly expanded medially, row of ventral spines present, not markedly stout; calcipala well expressed, pedisulcus barely evident ( Fig. 106 View FIGURES 103 – 106 ); tarsal claw partially covered by grappling pad of ca. 20 hooks, distinct basal tooth. Abdomen ( Fig. 107 View FIGURES 107 – 110 ): blackish brown; abdominal scale with long fine hairs, tergites markedly broad, tergites II–IV hirsute, less so on posterior others. Genitalia: ventral view ( Fig. 108 View FIGURES 107 – 110 ); ventral plate directed ventrally giving appearance of broadly concave apex, 1.5–2.0x wider than long, median keel well developed, vestiture of fine hairs, plate not sculpted laterally; anteromedial broad and slightly convex, basal arms fine, heavily pigmented, splayed apically ( Fig. 109 View FIGURES 107 – 110 ), paramere connectors well developed; median sclerite well expressed, broad and divided apically; parameres triangular, plate–like, strengthened laterally, spines as small spicules, not meeting medially; adeagal membrane with minute microtrichia; gonocoxa 2.0x longer than basal width, markedly sparse hairs; gonostylus approximately 2.0x longer than basal width, apically with 2–4 substantial terminal spines ( Fig. 110 View FIGURES 107 – 110 ).
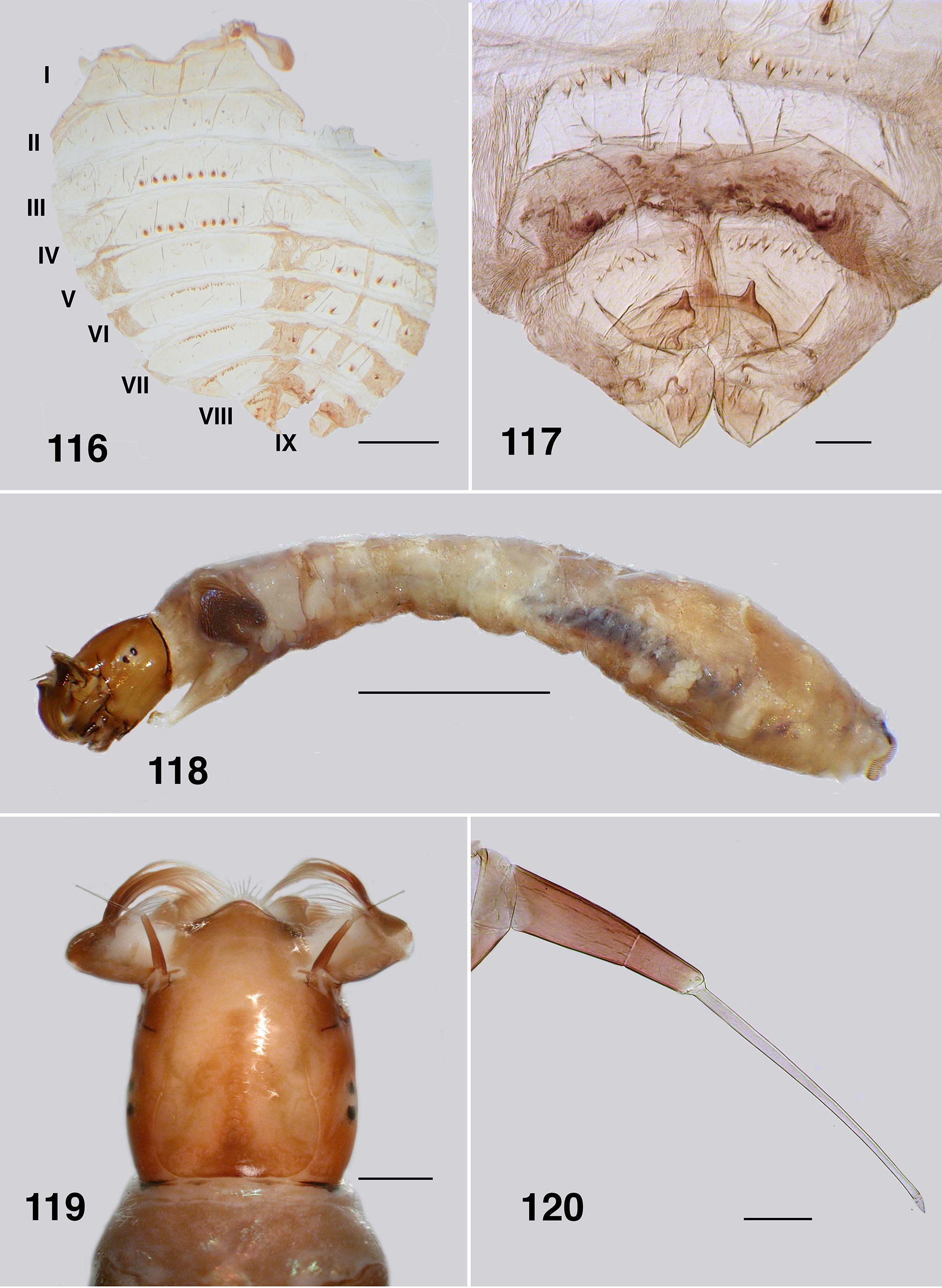
Pupa (based on numbers of specimens). Body: length, female 3.2–3.5 mm; male 3.2–3.3 mm ( Fig. 111 View FIGURES 111 – 115 ). Head: frontal cephalic plate lacking dorsal depression; ratio of basal width to vertex width of female 1:1.5, for basal width to length 1:1.5, rounded apically ( Fig. 113 View FIGURES 111 – 115 ), male ratios 1.0:1.5 and 1.0:2.0 respectively ( Fig. 114 View FIGURES 111 – 115 ), evenly tuberculate, frontal and facial setae present, but insubstantial, ocular spine absent. Thorax: Dorsum with very small tubercles, no pattern; dorsocentral setae substantial, spine–like and curled apically, other more lateral setae also spine–like in expression ( Fig. 115 View FIGURES 111 – 115 ). Gill ( Fig. 111, 112 View FIGURES 111 – 115 ): fundamentally of three flattened laminae, curled along their length, with concertina marks from packaging in the histoblast appearing as annulations ( Fig. 126 View FIGURES 121 – 126 , 127 View FIGURES 127, 128 ); anterior trunk 1.6–2.1 mm length (ca. 14 annulations), stub–like posterior lobe ca. 0.25 mm (ca. 6 annulations), ventral trunk ca. 0.6 mm in length (ca. 7 annulations); fine filaments, as such, absent. Abdominal armature ( Fig. 116 View FIGURES 116, 117 ): tergites I & II with 4–6 fine setae, lightly tuberculate; tergite III with four hooks per side, 3 or 4 other fine setae, no sternal hooks; tergite IV as for III; tergite V with poorly expressed spine comb, 4 hooks on sternum, laterally small plurites; tergite VI with spine comb and four hooks ventrally, one on small pleurite; tergite VII as for VI; VIII with a markedly poorly expressed spine comb anteriorly, spines reduced laterally; tergite IX with similar spines comb; terminal spines short, not sharp, grapnel hooks poorly expressed, as single hooks ( Fig. 117 View FIGURES 116, 117 ). Cocoon. Surface smooth, fabric coarsely woven, silk filaments obvious, medium brown; distinctly slipper–shaped fully covering pupa, not close fitted, with well defined anterior edge, ventral floor absent.
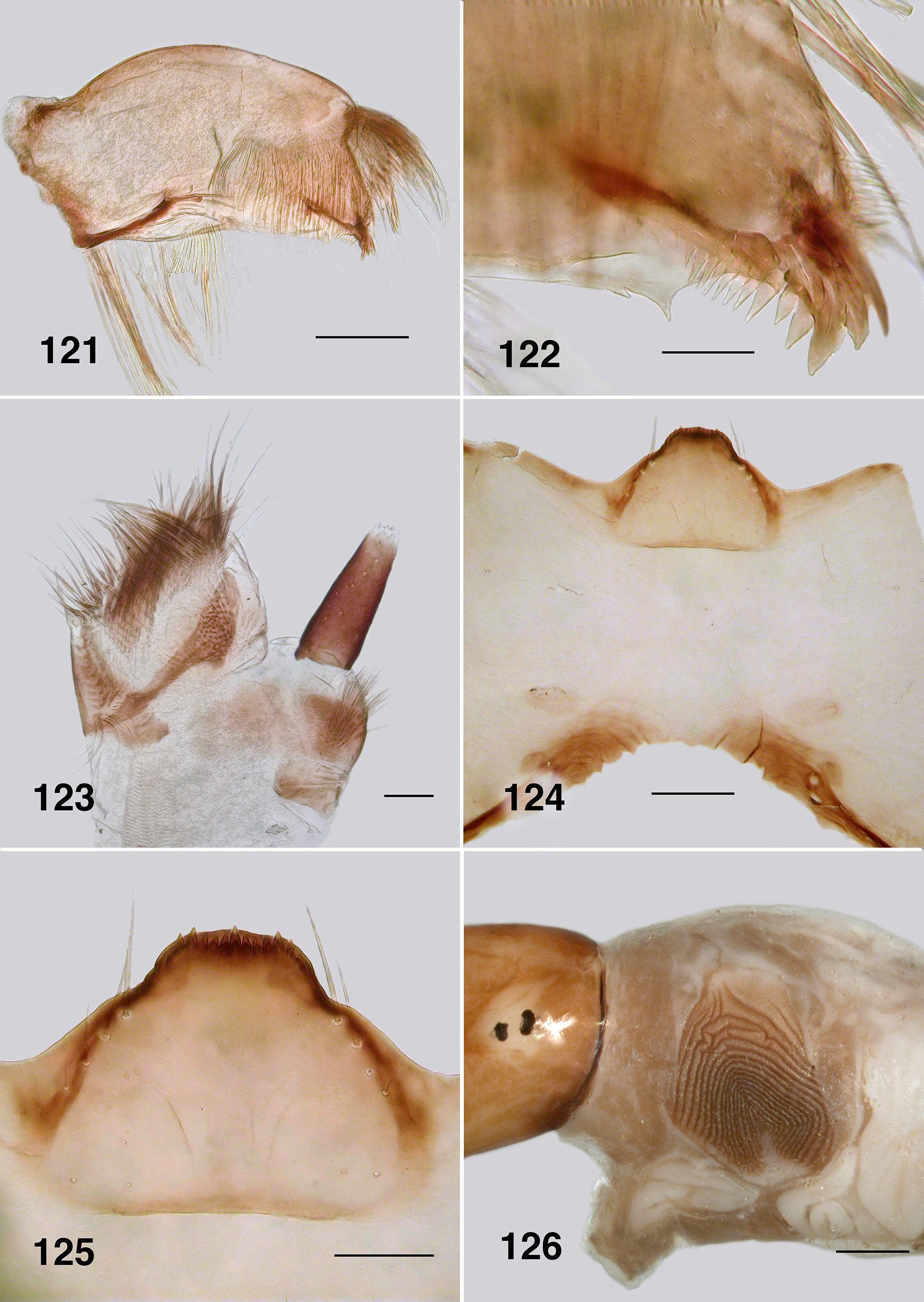
Larva (based on numbers of last instar larvae). Body (Fig. 118): overall yellowish gray, anterior abdomen of smaller diameter than thorax, expanded smoothly posteriorly; total length 5.8–6.5 mm. Head (Figs. 119): evenly mid brown, head spots positive, but not strongly pigmented; head length 0.73–0.86 mm, width 0.66–0.67 mm; distance between antennal bases 0.50–0.51 mm; lateral margins of head smoothly convex; cervical sclerites well developed and pigmented, elongated and fused to postocciput; anterolateral edges of apotome not distinctly pigmented; genae lacking darker 'eye brow' over stemmata. Antenna (Fig. 120): apical article pale, others dark brown; total length ca. 0.4 mm; well extended beyond labral fan stem; distal article subequal in length to other two articles; ratio of basal, medial and apical articles 1.0:0.6:2.0. Labral fan: relatively small; stem translucent; 56–60 fine rays, 0.32 mm in length, 0.005 mm in width; microtrichia as long as ray width, substantial, interspersed with 2 or 3 smaller microtrichia. Mandible ( Fig. 121, 122 View FIGURES 121 – 126 ): apical brush moderately developed; apical teeth not markedly extended; subapical teeth small, 8–9 substantial spinous teeth; mandibular sensillum and serrations often complex, but not markedly so, sometimes as just a simple pair (as shown). Maxilla ( Fig. 123 View FIGURES 121 – 126 ): lobe markedly cone–shaped, asymmetrical, palp subequal in length to lobe, well separated, 2x as long as basal width. Postgenal cleft ( Fig. 124 View FIGURES 121 – 126 ): essentially absent; ratio of hypostoma, bridge and cleft 1.0:1.6:0.3; suboesophageal ganglion not pigmented. Hypostoma ( Fig. 125 View FIGURES 121 – 126 ): ventral edge broadly domed–shaped, more or less covering 15 teeth; median tooth barely protruded beyond edge, sublateral teeth smaller and subequal in length, barely protruded beyond edge, lateral tooth larger and protruded, paralateral teeth smaller and sharp, variable expression, no others evident; no hypostomal serrations; four hypostomal setae per side. Postgenal bridge: pale and concolourous with genae. Thorax: ( Fig. 126 View FIGURES 121 – 126 ) anterior prothorax brown, remainder paler; pharate pupal gill broad, inverted V–shape, concertinaed ( Fig. 127 View FIGURES 127, 128 ). Abdomen: pale brown anteriorly, darker posteriorly; abdominal segments expanded smoothly; posteroventral tubercles present, but not markedly developed. Anal papillae: three simple lobes. Posterior proleg ( Fig. 128 View FIGURES 127, 128 ): rectal scales present in large numbers; anal sclerite median region well expressed with lateral interarm struts distinct, anterior arms slightly flared, shorter than posterior arms, posterior arms irregular in outline; accessory and pigmented semicircular sclerites absent. Posterior circlet: ca. 80 rows of 15–17 hooks (total ca. 1,250).
Etymology. Not detailed by Bigot (1888: 15), but apparently from Greek 'ανΘΡακίτης' or Latin ' anthracite ', meaning 'coal–like'; no doubt in reference to colour of the male and, less so, the female.
Types. The lectotype female is housed in the Natural History Museum, London, along with a male and a female paralectotype ( Crosskey & Lowry 1990: 203). Details of labeling unknown.
Material examined. Five tubes of ETOH material. Larvae, pupae, males, females. Label data:- [Paraustrosimuium/ anthracinum / (Bigot)/ det. Coscarón 84] [ Argentina Rio Negro / Bariloche 11-ix-84 / Coll. Coscarón] [Ex-Davies Collection,/ McMaster. 2011] [ UASM #/ 370852]. Larvae (damaged). Label data:- [ Paraustrosimulium anthracinum / Kerguen, Ao, Castillo, 21.9.84] [Ex-Davies Collection,/ McMaster. 2011] [ UASM #/ 370853]. Larvae (damaged). Label data:- [ Paraustrosimulium / anthracinum / (Bigot)/ det. H. Gyorkos - '85] [Bariloche, Ao Villa/ Con Bosca. 19.ix. 84 / Col. Coscarón] [Ex-Davies Collection,/ McMaster. 2011] [ UASM #/ 370854]. Males & females. Label data:- [Paraustrosimuium/ anthracinum / (Bigot)/ det. Coscarón 92] [ Chile. Magallanes / Isla Deslit/ 9-xi-82. Col. Lanfranco] [ UASM #/ 370855]. Larvae, pupae, male, female. Label data:- [Paraustrosimuium/ anthracinum / (Bigot)/ det. Coscarón 82] [ Chile. Chiloe/ rio Melitaba/ e/Huillinco/ y/ Chonchi/ 30-xi-92 / Col. Coscarón] [ UASM #/ 370856]. Slides, fourteen, all stages:- [Paraustrosimuium / anthracinum ] [ Chile / Rio Melitaba y/ Chonchi/ 30-xi-92 / Col. S. Coscarón] [ UASM #/ 370891-370904]; one, female [[ Chile. Magallanes / Isla Deslit/ 9-xi-82. Coll. Lanfranco] [ UASM #/ 370905].
Distribution. Widespread in southern South American Andes—Wygodzinsky & Coscarón (1973) give details. In short, in Chile P. anthracinum extends from northern Terra del Fuego, northwards to Malleco. In Argentine, from Islas de los Estado off the coast of Terra del Fuego, northwards to southern Neuquen Province.
Bionomics. Wygodzinsky & Coscarón (1962) note that for their material of P. anthracinum , the aquatic stages were found attached to dead vegetation trailing in the water of a large stream, near the edge and just below the surface—the population was monospecific. Specimens reported by Dumbleton (1960) from Navarino Island similarly came from a large stream, however, those collected by Wygodzinsky (1953) some 1,200 km farther north in the Province of Rio Negro, Argentine, were obtained from smaller water courses. Hernández et al. (2009: 196) record larvae from a murky water stream on Isla Victoria, Lake Guillelmo, near Bariloche and the few females collected from that region were not markedly anthropophilic. Coscarón & Coscarón Arias (2007) noted that P. anthracinum was anthropophilic.
Dates of collections by Wygodzinsky & Coscarón (1973: 192) ranged from October to February. This covers the Southern Hemisphere spring and early summer, but there is no information regarding water temperatures, nor altitude as such. Some of the collections included all stages, so P. anthracinum would appear to be a multivoltine species.
Remarks. Given the wide–ranging distribution of P. anthracinum , variation between populations is perhaps to be expected. The earlier descriptions give variation in numbers of terminal spines on the male gonostyli and we see differences in the width of the female frons (see above). A more detailed examination of the species, involving molecular analysis will probably reveal cryptic species.
Character comparisons. We here make character-state comparisons between Paraustrosimulium (as re- defined) and related simuliids. While it is beyond the scope of this paper to undertake a comprehensive character analysis of austral simuliids, the following discussion of states provide the basis for identifying possible synapomorphies of Paraustrosimulium , and for offering remarks about its close relationship to other taxa, in particular Austrosimulium , “ Cnephia ” pilfreyi and Cnesiamima atroparva .
Adults. Antenn a: An antenna with 9 flagellomeres is inferred to be the ground plan condition in the Simuliidae based on the distribution of that character-state throughout the family ( Adler et al., 2004). Austrosimulium typically has only 8 flagellomeres (rarely nine), as do the three species now assigned to Paraustrosimulium . In fact, the shared presence of 8 flagellomeres was the main criterion by which Edwards (1931) originally assigned anthracinum Bigot to Austrosimulium . Both “ Cnephia ” pilfreyi and Cnesiamima atroparva exhibit the plesiomorphic condition.
Frons: Females of Austrosimulium s.str. have a markedly broad frons, with the lateral margins diverging dorsally. Paraustrosimulium anthracinum has a narrower frons, but with similar margins ( Fig. 87 View FIGURES 86 – 91 ); P. colboi ( Fig. 2 View FIGURES 1 – 6 ) also has a comparatively narrow frons, but with margins not as divergent; P. obcidens has by far the narrowest frons of the three Paraustrosimulium species, with its lateral margins subparallel ( Fig. 46 View FIGURES 45 – 50 ). Mackerras & Mackerras (1948: 248) illustrated (their Fig. 9 View FIGURES 7 – 11 ) the female heads of A. ( Novaustrosimulium ) species A. bancrofti , A. pestilens , A. furiosum and A. mirabile . These are considerably closer in expression to Paraustrosimulium than to those of Austrosimulium (Austrosimulium) .
Mandible of female: Austrosimulium females have mandibular teeth only on the inner apical surface. Paraustrosimulium female mandibles have teeth on both sides of the apex, albeit poorly developed on the outer edge (e.g. Figs. 5 View FIGURES 1 – 6 , 49 View FIGURES 45 – 50 ); the mandible apex is also broadly triangular apically, unlike those of Austrosimulium . In their description of P. colboi, Davies & Györkös (1988) noted that teeth were present only on the inner surface of the mandible; however, given the poor quality of the material at their disposal, the outer teeth could easily have been overlooked. Mandibles of the females of “ Cnephia ” pilfreyi and Cnesiamima atroparva have teeth on both margins.
Maxillary palp: Austrosimulium (Austrosimulium) females tend to have the 3rd and 4th palpomeres more or less subequal in length, with the 5th palpomere 1.5x longer than the 4th. Paraustrosimulium colboi exhibits a similar condition ( Fig. 4 View FIGURES 1 – 6 ), whereas P. obcidens has a semispherical 3rd palpomere, a markedly small 4th palpomere, and with the 5th palpomere subequal in length to the 3rd ( Fig. 48 View FIGURES 45 – 50 ). The palp of Paraustrosimulium anthracinum ( Fig. 89 View FIGURES 86 – 91 ) is similar to that of P. colboi . Austrosimulium (Novaustrosimulium) females have a markedly narrowed 3rd palpomere, subequal in length to each of the more distal ones—a likely synapomorphy of that subgenus.
Cervical sclerites: All simuliid adult possess lateral cervical sclerites posteriorly on the neck. In Paraustrosimulium they are accentuated—markedly so in P. obcidens ( Fig. 45 View FIGURES 45 – 50 ) and P. anthracinum ( Fig. 86 View FIGURES 86 – 91 ). We consider this character state to be a synapomorphy of the genus.
Antepronotal lobe: Neither P. colboi ( Fig. 1 View FIGURES 1 – 6 ) nor P. obcidens (Figs. 43, 45) have noticeably hirsute antepronotal lobes, whereas those of P. anthracinum are markedly so, along with the postocciput of the head ( Figs. 86, 87 View FIGURES 86 – 91 ). This character is not well surveyed across austral simuliid taxa and is currently of limited phylogenetic value.
Wing: Spiniform setae on the costa of P. anthracinum are densely packed along the distal half, although relatively fine in expression ( Fig. 93 View FIGURES 92 – 96 ). In P. colboi and P. obcidens the spiniform setae are more widely-spaced and substantial (e.g. Fig. 8 View FIGURES 7 – 11 ). Paraustrosimulium anthracinum has spinous hairs on the distal portion of the R1 ( Fig. 92 View FIGURES 92 – 96 ), as does Cnesiamima atroparva . Such spines are absent from the other two species of Paraustrosimulium and Austrosimulium . Paraustrosimulium colboi and P. obcidens have the apices of veins R1 and Rs closely appressed or fused before joining C, as in Austrosimulium . The apices of veins R1 and Rs are not as closely appressed in P. anthracinum , although the latter vein deflects markedly towards the anterior margin shortly beyond its base.
Leg Spines: The ventral row of stout spines that typically occur on the hind basitarsus is absent from P. colboi , P. obcidens (e.g. Fig. 11 View FIGURES 7 – 11 ) and members of the ungulatum –species group of New Zealand Austrosimulium . The spines are present in most members of the australense –species group and in P. anthracinum , although in the latter species are longer and finer than is typical ( Fig. 95 View FIGURES 92 – 96 ).
Tarsal claw basal tooth: Females of Paraustrosimulium obcidens and P. anthracinum each have a tooth that is markedly expressed, blunt, and with a distinct notch between the tooth and heel ( Figs. 54 View FIGURES 51 – 55 , 96 View FIGURES 92 – 96 ). The female claw of Cnesiamima atroparva is markedly similar in overall form. In contrast, the tooth in females of P. colboi is essentially absent ( Fig. 12 View FIGURES 12 – 17 ). The claw tooth in New Zealand Austrosimulium is present only in members of the ungulatum species-group, although not as markedly developed as in P. obcidens and P. anthracinum , and with only certain species having a notch between the tooth and heel. This type of claw is also present in the Australian species of the ungulatum species-group (e.g., A. crassipes Tonnoir , A. cornutum Tonnoir ). Members of the mirable species-group (e.g. A. (A.) mirable , A. (A.) montanum ) possess a rather different claw from the others; specifically, a large basal tooth of different form that lacks both a notch and a heel. Species of subgenus Novaustrosimulium typically exhibit no expression of a tooth or heel, the only exception being Austrosimulium (N.) magnum , which possesses a small sharp tooth. Marked variation on basal tooth form makes this character suspect at higher phylogenetic levels, but has utility for characterizing groups of closely related species.
Genital fork: The three species of Paraustrosimulium have genital forks that are similar in overall form to those of the New Zealand Austrosimulium , although expression in the latter is rather varied ( Craig et al. 2012). Other aspects of the genital fork, such as broad anterior arm, short broad lateral arms with variously expressed 'knee bends', small apodeme, and enlarged posterolateral lobes are similar to those of the ungulatum –species group. Such also occurs in the bancrofti – species group of Novaustrosimulium —but less so in the furiosum –species group. These variations notwithstanding, the genital fork of Austrosimulium and Paraustrosimulium differ from those of all other simuliids by the combination of a lack of intense pigment and the comparatively short and wide anterior- and lateral arms. In contrast, the genital forks of “ Cnephia ” pilfreyi and Cnesiamima atroparva is more distinctly pigmented and proportions of the anterior- and lateral arms are typical of other simuliids.
Spermatheca: None of the three species in Paraustrosimulium have patterning on the spermathecal surface, and all have sparse internal acanthae. Paraustrosimulium obcidens has considerable pigmentation down the sperm duct, but no sculpting at the junction ( Fig. 58 View FIGURES 56 – 59 ). Paraustrosimulium colboi has pigmentation for a short distance along the spermathecal duct and minor sculpting of the junction ( Fig. 16 View FIGURES 12 – 17 ). Paraustrosimulium anthracinum has neither pigmentation extended along the duct nor sculpting or clear area ( Fig. 97 View FIGURES 97 – 101 ). Most species of New Zealand Austrosimulium have a clear area at the duct junction, the only exception being A. unicorne Dumbleton of the ungulatum –species group. For all Australian Austrosimulium , for which material is available, the spermathecae are smooth externally, lack acanthae, and have a large clear area at the duct junction with sculpting at the pigmented edge of the spermatheca.
Cercus and anal lobe: The cercus of all three Paraustrosimulium species is rounded apically—a common condition in the Simuliidae . The anal lobe P. colboi and P. obcidens is angulate proximally, similar in form to those of Gigantodax species ( Wygodzinsky & Coscarón 1989). In contrast, the anal lobe of P. anthracinum is tapered proximally.
Male genitalia: In New Zealand Austrosimulium (Austrosimulium) the parameres are either absent or represented at most by a slender twisted rod of cuticle. Further, parameral spines are entirely lacking from such species ( Craig et al. 2012). There is little or no mention of these character states by Dumbleton or the Mackerras'; however, examination of Australian Austrosimulium (Austrosimulium) species (Craig per. obs. 2014) reveals they are similar to New Zealand species, as described above. The subgenus Novaustrosimulium also has a weakly expressed paramere, but small spines are present in members of the furiosum –species group. These were illustrated by Mackerras & Mackerras (1949: 393. their Fig. 13c View FIGURES 12 – 17 for A. (N.) crassipes). A. bancrofti has small parameres, but lacks spines (DAC pers. obs.). The ventral plate of members of the furiosum –species group possess a keel, and thus resemble those of Paraustrosimulium ; the angulate gonostyli are also shared between members of these two taxa. In contrast, the gonostyli of Austrosimulium (Austrosimulium) and members of the bancrofti –species group of Novaustrosimulium are more tapered.
Pupa. Gills: The gills of P. colboi and P. obcidens (Fig. 26, 70) are inflated, tubular, structures that are similar in form to those of Cnesiamima atroparva , except for the presence of sparse fine filaments in the latter species ( Coscarón 1991: 74, his Fig. 14 C View FIGURES 12 – 17 ). The gill of “ Cnephia ” pilfreyi is also an inflated, tubular, structure, although consisting of 6 filaments (as opposed to a single, large, cornuate structure, as in the previously mentioned species). The gills of P. anthracinum , while superficially similar in appearance to the other two species of Paraustrosimulium , are actually not tubular in form. Rather, they consist of flattened elongated curled laminae. The P. anthracinum gill appear to be more similar in form to that of Simulium (Hemicnetha) canadense ( Hearle 1935: 14–15; Adler et al. 2004: 538) or to those of certain members of the cormonsi –species group of Gigantodax ( Wygodzinsky & Coscarón 1989) . While the external structure of gills seems to be rather varied in Paraustrosimulium , the way they are packaged within the gill histoblast of the last instar larva appears to be similar. When fully inflated, the pupal gill of P. obcidens is ca. 0.90 mm long with a maximum diameter of 0.09 mm ( Fig. 70 View FIGURES 68 – 73 ). The dimensions of the same gill when contained within the histoblast of a late last-instar larva is ca. 0.58 mm in length and 0.21 mm maximum breadth—a packaging ratio of ca. 1:2 for the length. This is achieved by each distal annulation being concertinaed sequentially into a larger more proximal annulation. The packaging of gills is further enhanced because each annulation is pleated laterally ( Fig. 82 View FIGURES 80 – 84 ), further reducing the diameter of each annulation—an elegant piece of biological engineering. Similar packaging is seen to the gill histoplast of pharate pupal P. anthracinum ( Figs. 111, 112 View FIGURES 111 – 115 , 126 View FIGURES 121 – 126 , 127 View FIGURES 127, 128 ). Although the packaging of pupal gills has not been widely surveyed in the Simuliidae , it seems possible that the mechanism described above is synapomorphic—at least for Paraustrosimulium and perhaps also for Paraustrosimulium + Cnesiamima .
Cephalic plate: A character of considerable taxonomic utility for Austrosimulium is a marked depression of the vertex in the cephalic plate of the pupa. Craig et al. (2012) considered this state to be a synapomorphy of the australense –species group. It is absent from both members of the ungulatum –species group and the three species of Paraustrosimulium .
Abdominal armature: Pupae of Austrosimulium and Paraustrosimulium have reduced abdominal armature, in particular ventral hooks which are absent in certain species of Austrosimulium and P. colboi . The terminal spines are also markedly reduced in members of those two genera, as well as in Cnesiamima and “ Cnephia ” pilfreyi. In Paraustrosimulium and Cnesiamima , the role of holding onto cocoon silk has evidently been assumed by a series of terminal grapnel hooks ( Figs. 31 View FIGURES 30 – 31 , 73 View FIGURES 68 – 73 ). Similar grapnel hooks are present in the pleura of abdominal segments VIII and IX of pupal Metacnephia Crosskey ; however, this is almost certainly an independently derived state based on the relatively derived phylogenetic position of that genus ( Adler et al., 2004). The stiff apically curved dorsocentral setae of the thorax (e.g. Fig 69 View FIGURES 68 – 73 ) perhaps serve a similar role in Paraustrosimulium and Cnesiamima , but are comparatively less-well developed in Austrosimulium . Spine combs on tergite IX of Austrosimulium and Cnesiamima are absent, and only weakly expressed in Paraustrosimulium ( Figs. 31 View FIGURES 30 – 31 , 73 View FIGURES 68 – 73 )—an unusual character- state that, in combination with the reduced size of the terminal spines, is considered synapomorphic for these three segregates.
Cocoon: The three Paraustrosimulium species all possess a more–or–less similar slipper-shaped cocoon. Those of P. anthracinum and P. obcidens ( Fig. 68 View FIGURES 68 – 73 ) have a well-defined anterior opening, no floor, and a well- defined ventral edge on the substrate. That of P. colboi is more substantial (Fig. 26) and has a distinct floor. Similar cocoon shapes can be seen in Austrosimulium and Cnesiamima . That of “ Cnephia ” pilfreyi differs in that it has a raised anteroventral collar, giving the cocoon a distinctive shoe-shape ( Davies & Györkös, 1988).
Larva. Antenna: Larval antennal proportions of Paraustrosimulium species are all similar, with the ratios of basal, median and distal antennomeres 1.0:0.6:2.0. (Figs. 34, 76, 120). Antennae of New Zealand Austrosimulium larvae have somewhat variable proportions for the basal two antennomeres; but all have an elongated distal antennomere that is conspicuously longer than the combined length of the proximal antennomeres ( Craig et al. 2012: their Figs. 367–383). The only Austrosimulium species in which the distal antennomere is shorter than the combined length of the proximal antennomeres are members of the subgenus Novaustrosimulium . In that segregate, the two proximal antennomeres are of approximately equal length and the distal antennomere is shorter than as described above, with ratios ranging from 1.0:1.1:1.5 to 1.0:0.8:1.1. The elongate condition of the distal antennomere is clearly derived within the Simuliidae . The only other austral simuliids exhibiting a similar condition is Cnesiamima and “ Cnephia ” pilfreyi. It seems possible that an elongate distal article could be synapomorphic for Paraustrosimulium , Austrosimulium , Cnesiamima and “ Cnephia ” pilfreyi; albeit, implying a reversal in A. ( Novaustrosimulium ).
Mandible: Mandibles of Australian Paraustrosimulium View in CoL View at ENA larvae have apical teeth that are finely expressed and narrowly projected apically (e.g., Fig. 35 View FIGURES 35 – 40 ). Such is also expressed in the Australasian Austrosimulium s.str. and A. ( Novaustrosimulium ). The apical teeth in P. anthracinum are comparatively more substantive. The mandibular sensillum and serration ( Craig 1977) in all Austrosimulium larvae are complex ( Craig et al., 2012), unlike the simple projections in the three Paraustrosimulium species ( Figs. 35 View FIGURES 35 – 40 , 78 View FIGURES 74 – 79 , 122 View FIGURES 121 – 126 ).
Hypostoma: All three species of Paraustrosimulium have the ventral wall of the hypostoma extended anteriorly such that it obscures the hypostomal teeth, which in turn are not grouped in a distinctive fashion ( Figs. 38 View FIGURES 35 – 40 , 81 View FIGURES 80 – 84 , 125 View FIGURES 121 – 126 ). In this respect, the hypostoma of Paraustrosimulium is virtually identical to those of Austrosimulium and “ Cnephia ” pilfreyi. The hypostomal teeth of Cnesiamima atroparva are also obscured by the ventral wall of the hypostoma ( Coscarón 1985: 69); however, unlike the previously mentioned taxa, the outermost sublateral, lateral, and paralateral teeth are clustered onto a prominent lobe. The relatively extended ventral hypostomal wall might constitute evidence of common ancestry among these taxa.
Postgenal cleft: The cleft is essentially absent in P. anthracinum ( Fig. 124 View FIGURES 121 – 126 ) but relatively well developed in P. colboi ( Fig. 37 View FIGURES 35 – 40 ) and P. obcidens ( Fig. 80 View FIGURES 80 – 84 )—similar in expression to the New Zealand Austrosimulium . The cleft is of variable expression in the Australian Austrosimulium , for example ranging from absent in A. (A.) montanum to well developed in Novaustrosimulium species. The postgenal cleft is absent from both Cnesiamima atroparva and “ Cnephia ” pilfreyi.
Anal sclerite interarm strut: This character-state is clearly derived within the Simuliidae based on its absence from members of the Parasimuliinae and Prosimuliini . Tonnoir (1925) used the presence of interarm struts, in part, to characterize members of the genus Austrosimulium . However, that state is now known to be more widely distributed among simuliine genera, including Paraustrosimulium and Gigantodax . In larvae of Austrosimulium and P. anthracinum ( Fig. 128 View FIGURES 127, 128 ) the interarm struts appear to be the lateral strengthened edge of a broad medial region that bridges the dorsal and ventral arms; in most examples, the cuticle between the struts is pigmented. The condition in P. colboi ( Fig. 40 View FIGURES 35 – 40 ) and P. obcidens ( Fig. 83 View FIGURES 80 – 84 ) is similar except the interarm struts are somewhat more closely approximated (giving the sclerite a more distinctive X–shaped appearance), and the cuticle between the interarm struts is sparsely pigmented. Superficially, therefore, the laterally strengthened edges appear more definitely as independent struts, as opposed to being part of a more integrated medial sclerotized area between the dorsal and ventral arms. In this respect, the interarm struts in P. colboi and P. obcidens more closely resemble the condition in Gigantodax than in P. anthracinum and Austrosimulium . However, phylogenetic studies based on morphological (Gil–Azevedo & Maia–Herzog 2007: 60) and molecular ( Moulton, 2003) datasets reveal that Gigantodax is distantly related to Paraustrosimulium and Austrosimulium . Accordingly, interarm struts must have evolved independently at least twice within the Simuliidae . Coscarón & Coscarón Arias (2007: 94) remarked that the anal sclerite of Cnesiamima was without or with “only faint” interarm struts (cf. their figure 22N View FIGURES 18 – 23 ), perhaps suggesting a close relationship with Austrosimulium and Paraustrosimulium . Davies & Györkös (1988) reported that interarm struts were absent from the anal sclerite of “ Cnephia ” pilfreyi; however, their material was badly bleached so it is conceivable the arms are actually present but weakly expressed. Examination of more freshly collected material is needed to confirm the presence or absence of interarm struts in “ C. ” pilfreyi.
Semicircular sclerite: Homologies of the semicircular sclerite that surrounds the circlet of hooks in many Austrosimulium species is of similar concern to the interarm struts. While the presence of this sclerite appears to be of considerable taxonomic value in Austrosimulium , similar structures occur in larvae of other taxa, such as Parasimulium Malloch ( Adler et al., 2004) , Crozetia Davies ( Craig et al. 2003) , Gigantodax ( Wygodzinsky & Coscarón, 1973) and Simulium (Gomphostilbia) palauense Stone ( Takaoka & Craig 1999) . Dumbleton (1973: 563) provided examples of the varied forms of semicircular sclerite known at that time. Reasons for not considering these structures to be homologous, in all instances, include: (1) differences in expression of the junction of the semicircular sclerite to the ventral arm of the anal sclerite and, (2) semicircular sclerite expression is merely a product of the degree of sclerotization and pigmentation of the ring of cuticle that surrounds and supports the outer edge of the circlet of hooks ( Fig. 83 View FIGURES 80 – 84 ). Apparently, such a ring (whether darkly sclerotized or not) is present in all simuliid larvae ( Craig et al. 2012: 48). Accordingly, while the presence of a particularly expressed semicircular sclerite may prove to be synapomorphic for a given lineage, interpretations of homology must be made with caution.
| UASM |
University of Alberta, E.H. Strickland Entomological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paraustrosimulium anthracinum (Bigot)
| Craig, Douglas A., Moulton, John K. & Currie, Douglas C. 2017 |
Simulium moorei
| Silva Figueroa 1917: 30 |
Simulium (Austrosimulium) moorei
| Silva Figueroa 1917 |
Simulium moorei
| Silva Figueroa 1917 |
Simulium moorei
| Silva Figueroa 1917 |
Simulium anthracinum
| Bigot 1888: 15 |
Simulium anthracinum
| Bigot 1888 |
Simulium (Austrosimulium) anthracinum
| Bigot 1888 |
Simulium (Austrosimulium) anthracinum
| Bigot 1888 |
Prosimuliini
| Latreille 1802 |