Oxyporus ( Oxyporus ) maxillosus Fabricius, 1793
|
publication ID |
https://doi.org/10.37520/aemnp.2020.014 |
|
publication LSID |
lsid:zoobank.org:pub:BE18A83D-CDFC-4B02-82E8-A50E66E32C27 |
|
DOI |
https://doi.org/10.5281/zenodo.3811865 |
|
persistent identifier |
https://treatment.plazi.org/id/341BD143-FF9E-7478-FC8B-FCEB692FFF9C |
|
treatment provided by |
Valdenar |
|
scientific name |
Oxyporus ( Oxyporus ) maxillosus Fabricius, 1793 |
| status |
|
Oxyporus ( Oxyporus) maxillosus Fabricius, 1793 View in CoL ( Figs 1, 4 View Figs 1–6 , 7–27 View Figs 7–14 View Figs 15–18 View Figs 19–27 )
Material examined. 28 third instar larvae ( NHMD): RUSSIA: PRI- MORSKY TERRITORY: Arboretum of the Gornotaezhnaya Station FEB RAS, 43.6945498°N, 132.1520375°E, 11.ix.2018, from Armillaria sp., A. Tokareva leg. Two adults and 26 larvae were reared by A. Tokareva (rearing R28) from eggs collected in the fungus. GoogleMaps
DNA bardcoding. 1 third instar larva ( NHMD 620702: GenBank Accession No.: MN 508943 View Materials ) and one associated adult ( NHMD 620703: GenBank Accession No: MN 508942 View Materials ).
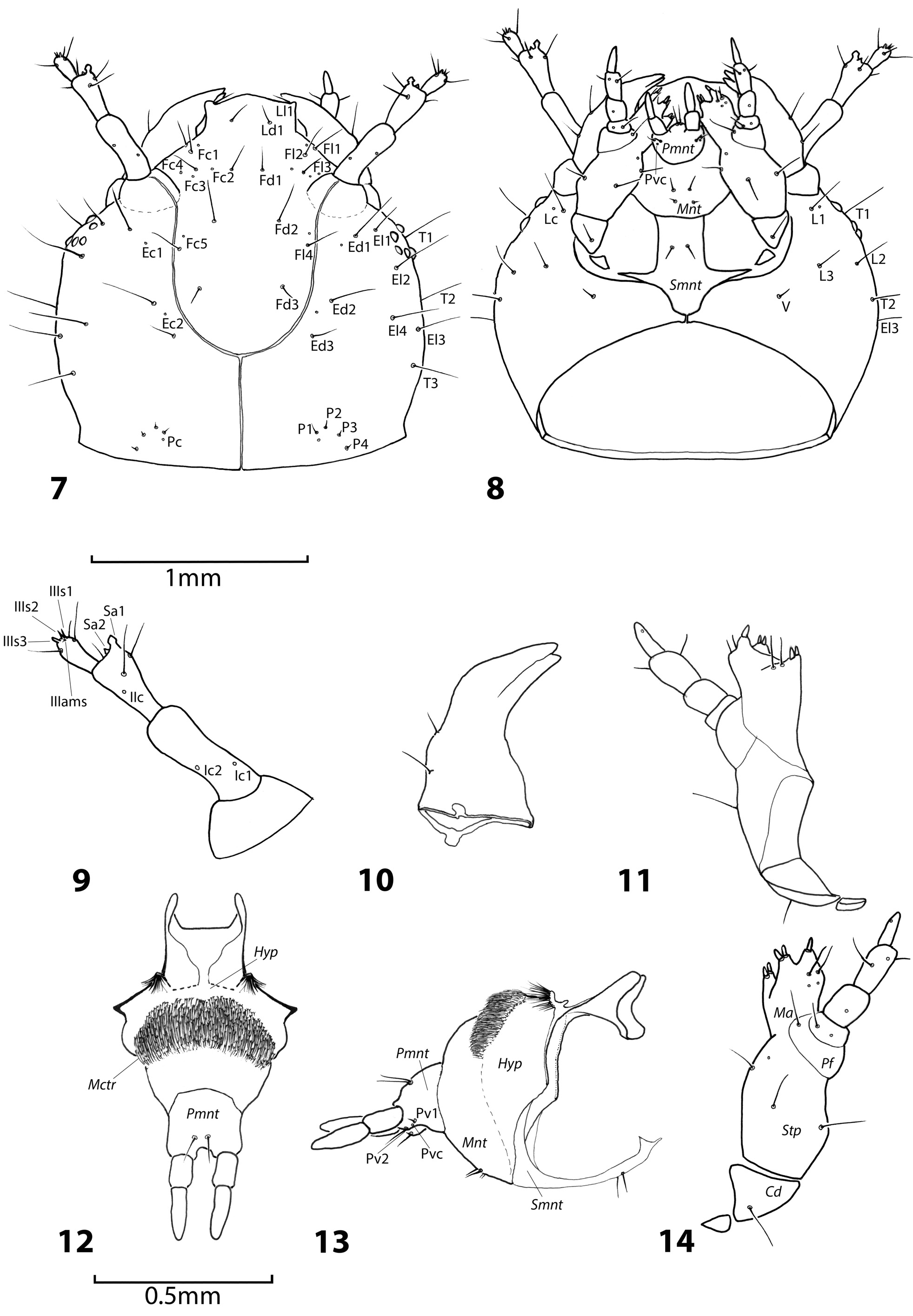
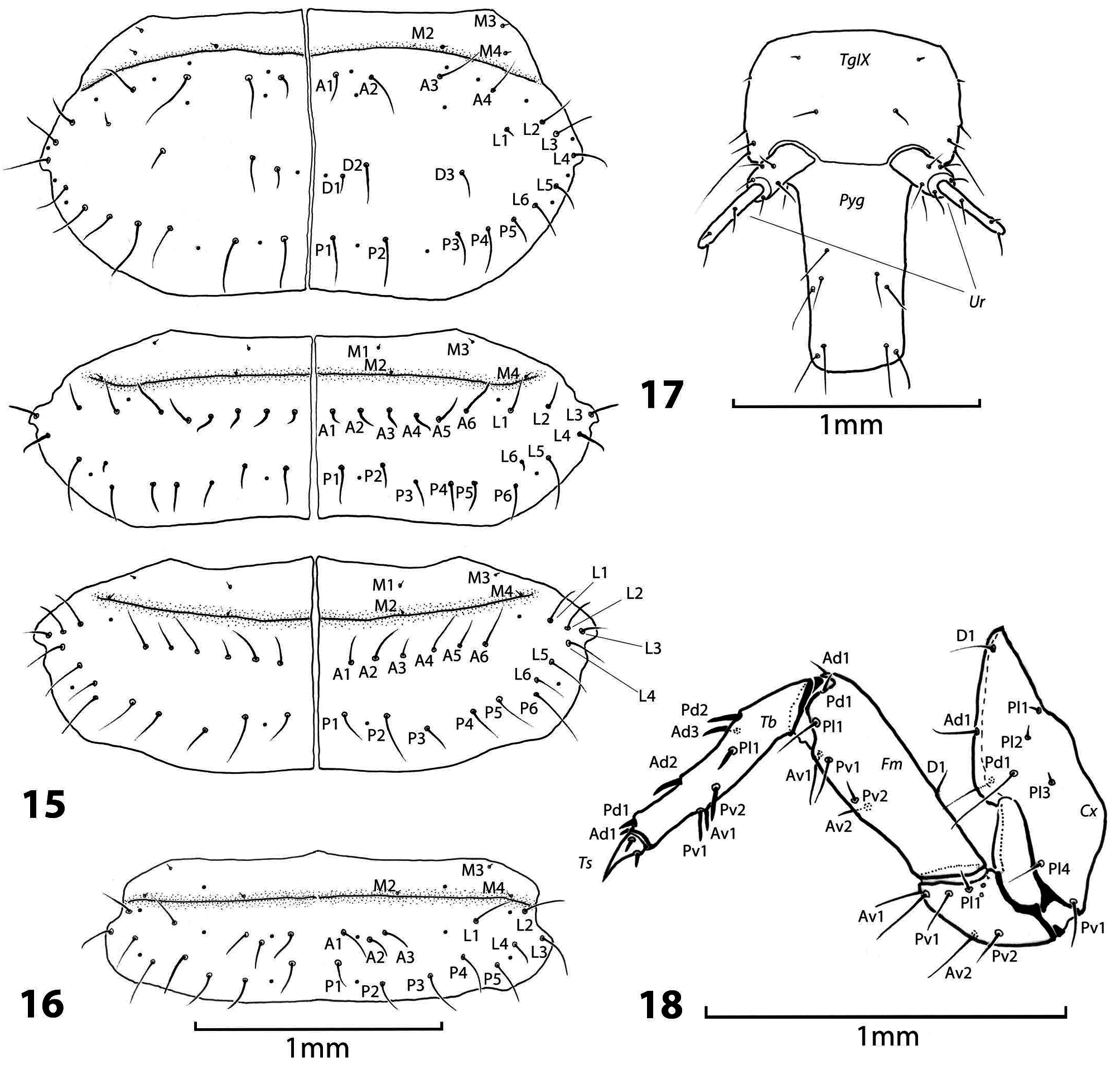
Diagnosis. Larva of O. maxillosus differs from all other described Oxyporus larvae by the following set of chaetotaxy characters: five posterior ( P) setae on each side of thoracic tergite I and six lateral ( L) setae on each side of thoracic tergite II. Oxyporus maxillosus larva further differs from O. procerus by having three membrane setae on each side before thoracic tergite I ( M2–M4); four anterior setae ( A1–A4) and five posterior setae ( P1–P5) on each side of thoracic tergite I, six lateral setae ( L1–L6) on each side of thoracic tergite II. From O. melanocephalus it differs by the following characters: two transversal setae on each side of the head capsule ( T1–T2); three membrane setae on each side before thoracic tergite I ( M1–M3); four anterior ( A1–A4), three discoidal ( D1– D3), six lateral ( L1–L6), and five posterior setae ( P1–P5) on each side of thoracic tergite I; six anterior ( A1–A6), six lateral ( L1–L6), and six posterior setae ( P1–P6) on each side of thoracic tergite II; six anterior ( A1–A6), six lateral ( L1–L6), and six posterior setae ( P1–P6) on each side of thoracic tergite III; three anterior ( A1–A3), four lateral ( L1–L4), and five posterior setae ( P1–P5) on each side of abdominal tergite ( Table 3 View Table 3 ).
Redescription. Eggs (n = 28). Early eggs uniformly white; length 1.1–1.2 mm; later turning dark yellow and larger, with visible larval mandibles under chorion surface, length 1.3–1.5 mm.
First instar larva. White, almost translucent, with reddish brown ocelli and mandibles.
Third instar larva. Head capsule yellowish brown with mosaic of darkened pits arranged in series on frons and occiput; thoracic and abdominal tergites uniformly greyish brown. Body length 10.3–13.1 mm; head length: 1.2–1.5 mm; head width: 1.3–1.4 mm.
Head. Oval, broadened at base ( Figs 7, 8 View Figs 7–14 ). Nasale with two pairs of setae of medium length ( Ll1, Ld1) on dorsal surface. Frontal setae arranged in vertical rows Fd1–Fd3 and Fl1–Fl4. Occipital group consists of microsetae P1–P4 and one campaniform sensillum. Epicranial setae arranged in rows on each side: Ed1–Ed3, El1–El4, T1–T3, L1–L3, V1. Head capsule with 52 setae in total. Campaniform sensilla (c. s.) present on head capsule: Fc1–Fc5, Ec1, Ec2, Pc ( Figs 22, 23 View Figs 19–27 ), Lc ( Fig. 24 View Figs 19–27 ). Antennomere I with two campaniform sensilla dorsally ( Ic1, Ic2) in basal portion and two ventrally ( Ic3, Ic4) in apical portion ( Fig. 8 View Figs 7–14 ). Mandibles with two setae on outer side. Maxillary palpi with palpifer as in general description; palpomere II with two setae and one campaniform sensillum. Labium as in general description; prementum with two setae and a campaniform sensilum at the base of each labial palp: Pv1, Pv2, Pc ( Figs 9–14 View Figs 7–14 ).
Thorax. Membrane anterior to pronotum with three pairs of microsetae ( M2, M3, M4 as interpreted in GOODRICH & HANLEY 1995 for mesonotum and metanotum: figs 9A, B, C). Pronotal tergite with anterior, discoidal, lateral, and posterior rows A1–A4, D1–D3, L1–L6 and P1–P5. Membrane anterior to mesonotum with four pairs of microsetae M1, M2, M3, M4. Mesonotum with anterior, lateral, and posterior ( A1–A6, L1–L6, P1–P6) setae. Metanotum with setation as on mesonotum ( Fig. 15 View Figs 15–18 ).
Legs. Tarsungulus with two spine-shaped, short setae. Tibiotarsus with five spine-shaped, short setae on dorsal side, two on lateral side, and three on ventral side, nine setae in total. Femur with three setae on ventral side, two on lateral side, three on dorsal side, eight setae in total. Trochanter with three setae near Tr–Fe joint of which medial seta twice as long as each neighboring seta, two setae medially, one short thin seta near coxal joint on each lateral side, and one short thin seta near Cx–Tr joint dorsally, in total eight setae. Coxa with 18 setae, including several basal microsetae ( Fig. 18 View Figs 15–18 ).
Abdomen. Membrane anterior to abdominal tergite I with three pairs of microsetae ( M2–M4). Abdominal tergite I with anterior, lateral, and posterior rows: A1–A3, L1–L4, P1–P5. Last abdominal tergite with four setae on dorsal side and three setae on each lateral side on posterior angles. Urogomphi as in generic description. Pygopod with asymmetrically arranged 18 setae ( Figs 16, 17 View Figs 15–18 ).
Development. According to SCHEERPELTZ & HÖFLER (1948) who described the development of the European O. maxillosus reared from the cluster of 30 eggs found in Pholiota lucifera (Lasch) Quél. at room temperature in laboratory, the life cycle takes 15 days from egg to the third moult (i.e. the moult to pupa). They provided the following data for the duration of each stage: egg: 48 hours; instar I: 4 days, instar II: 5 days; instar III: 5 days. Our field data show three larval instars as well and the life cycle taking 11–12 days from egg to imago (egg: 5–6 hours; instar I: 1–2 days, from instar II through instar III to prepupa: 6 days; prepupa: 1 day; pupa: 6–7 days). Duration of the second and third larval instars is unknown. Observed host fungi of larvae. Cortinariaceae : Cortinarius sp.; Physalacriaceae : Armillaria sp.; Strophariaceae : Pholiota sp.; Suillaceae : Suillus americanus (Peck) Snell. Observed host fungi for adults. Boletaceae : Leccinum holopus (Rostk.) Watling ; Cortinariaceae : Cortinarius sp.; Physalacriaceae : Armillaria sp.; Fomitopsidaceae : Laetiporus sp.; Strophariaceae : Kuechneromyces sp., Pholiota aurivella (Batsch) P. Kumm. , Pholiota sp.; Pleurotaceae : Pleurotus ostreatus (Jacq.) P. Kumm. , Pleurotus pulmonarius (Fr.) Quél. ; Suillaceae : Suillus americanus (Peck) Snell , Suillus sp.
Biology observations. Larvae were reared from eggs collected from chambers in the fruit body of Armillaria sp. with a female which presumably laid them. According to our observations, usually many individuals live together in a bracket of fungi like Pholiota sp., where one to three females can simultaneously be found in one cap where they are guarding their eggs in their respective chambers. Every female builds a chamber with a channel inside the cap. Each chamber may contain 10– 31 eggs. Sometimes one female builds two chambers with e.g. four and six eggs in each. Mothers remain in a chamber with their eggs or first instar larvae until the latter moult to the second instar and start moving out from the chambers, tunneling through the fungal fruit body. Presumably they do so to protect their brood from predatory myriapods, Bolitobius ( Staphylinidae ) species and other Oxyporus females, which compete for the room, as earlier suggested by SETSUDA (1994). Upon moulting into the third instar, larvae become pink, and shortly after they stop feeding and start to dig into the soil for pupation. Once a larva stops digging, it makes a pupation chamber, where it soon becomes quiescent until moulting into the adult (prepupal stage). Usually one or two females of O. basiventris Jarrige, 1948 or O. aokii Dvořák, 1956 were found in the same fungal bodies with O. maxillosus during field observations in Russian Far East.
| RAS |
Union of Burma Applied Research Institute |
| MN |
Museu Nacional, Universidade Federal do Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Oxyporus |