Oryzias eversi, Herder & Hadiaty & Nolte, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.5349994 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87E5-FF91-1119-FC2F-F865FAC20AF7 |
|
treatment provided by |
Tatiana |
|
scientific name |
Oryzias eversi |
| status |
sp. nov. |
Oryzias eversi View in CoL , new species
(English common name: Evers’ ricefish) ( Figs. 1–3 View Fig View Fig View Fig )
Material examined. — Holotype – MZB 20780 (35.8 mm SL), male, Indonesia, Sulawesi: South Sulawesi Province, Tana Toraja; Salo
Sadang drainage, stream close by the village Tilanga, about 8 km S of Rantepao; 3°02.126S, 119°53.232E, elev. 859 m; coll. H.-G. Evers, J. Christian, P. Debold, T. Heinrich, 24 Sep.2010.
Paratypes – All collected with the holotype: MZB 20781, 1 female (28.0 mm SL), ZFMK 44938 About ZFMK , 1 male (36.2 mm SL), ZFMK 44939 About ZFMK , 1 female (34.9 mm SL), USNM 406817 About USNM , 1 male (33.5 mm SL), USNM 406818 About USNM , 1 female (38.4 mm SL) .
Non-type material – ZFMK 44940-44943 About ZFMK , 4 immature specimens (16.8–21.6 mm SL), collected with the holotype .
Diagnosis. — Oryzias eversi is distinguished from all other Adrianichthyidae in Sulawesi by the following combination of characters: 17–18 (19) fin rays in the anal fin; 10–12 fin rays in the dorsal fin; 33–36 scales along lateral midline; ½14 transverse scale rows at dorsal fin origin; 30–32 (33) total vertebrae; small eye size relative to head length (28.2–35.5% HL); absence of dark bluish or steel blue body colouration or brilliant red marks in both sexes; conspicuous blackish courtship colouration of males, including a blackish belly and posterior lateral body, presence of 6–9 blackish lateral bars, and presence of a narrow black line on a light brown background on dorsal surface; i,4/5,i principal caudal fin rays; and a conspicuous pelvic brooding behaviour associated with sexually dimorphic body depth and pelvic fin length.
Description. — See Fig. 3 View Fig for general appearance in lateral view, Fig. 2 View Fig for radiographs of male holotype and a female paratype, and Table 1 for morphometric data. Body compressed laterally, slender to somewhat deep-bodied, body depth 18.9–24.6% SL. Females with a pronounced abdominal concavity between pelvic fins and anal fin, covered by long (18.9–19.6% SL) pelvic fins. Mouth terminal, lower jaw extends slightly beyond upper jaw. No external teeth. Ventral body profile arching from head to anal fin origin; body depth at anal fin origin smaller in females than in males (18.9–22.1 vs. 23.9–24.6% SL). Dorsal body profile almost straight from nape to dorsal fin. Dorsal surface of head nearly straight to slightly convex just anterior the orbit. Head small to moderate, head length 28.4–30.7% SL. Eyes moderate to small, 28.2–35.5% HL. Orbits not or only slightly projecting beyond dorsal surface of head. Caudal peduncle 1.1–1.6 times longer than deep; caudal peduncle length 12.8–17.9% SL, caudal peduncle depth 10.4–12.0% SL.
Genital papilla small and tubular in males, large, bilobed and rounded in females. Scales: 33–36 cycloid scales along lateral midline, ½14 transverse rows at dorsal fin origin. Dorsal fin with 10–12 rays, its origin at vertical through anal fin rays 8–11. Dorsal fin rounded but small, not reaching caudal base in females, rounded and long, with pronounced individual fin rays, reaching or extending caudal base in males. Anal fin with 17–18 (19) rays, short and straight to slightly concave with rounded tips in females, with pronounced individual fin rays, reaching or extending caudal base in males. Pelvic fin with 6 rays, the last ray is connected to the body by a membrane over half of its length in males, whereas such a membrane is lacking in females. Pectoral fin with 10 (9) rays, reaching to (females) or slightly beyond (males) pelvic-fin origin. Caudal fin truncate, with i,4/5,i principal caudal-fin rays; 6–7/6–8 procurrent caudal-fin rays.
Live colouration. — See Fig. 3 View Fig . Body whitish grey to light yellowish-brown with greenish sheen. Belly and throat white. Males in breeding mood with 6–9 blackish bars on lateral body, clearly distinct anterior to anal fin but less conspicuous in posterior body due to blackish to black background colouration. Males in breeding mood with blackish belly. Dorsal surface of head blackish, extending posteriorly as narrow black dorsal stripe on light cream- coloured background to dorsal fin; the black dorsal stripe may fade entirely. Opercle with silver bluish sheen. Iris golden with iridescent blue sheen. Fins hyaline, rays light cream coloured. Males in brooding mood with blackish to deep black dorsal, anal and pelvic fins, and blackish caudal fin with a narrow black margin. Pectoral fin hyaline, slightly blackish in males in breeding mood.
Colouration in preservative. — See Fig. 1 View Fig . In females and immature males, lateral body yellowish brown to grey, in mature males dusky grey to blackish. Males with 6–9 faint blackish bars on lateral body, females without such blackish bars or dusky grey to blackish colouration, but with a faint black lateral stripe on lateral midline, extending from uppermost posterior extremity of opercle to caudal base. Belly blackish grey in males, yellowish brown in females. Throat light yellowish brown. Dorsal surface of head brown to blackish, extending posteriorly as narrow blackish dorsal stripe to dorsal- and caudal fin. In females, fins dusky grey hyaline to light brown. In males, unpaired fins grey to blackish, with black caudal fin margins. Male pelvic fins blackish, male pectoral fins hyaline grey.
Sexual dimorphism. — Females grow slightly larger than males (maximum SL recorded: 36.2 mm in males, 38.4 mm in females) and differ in colouration in that they lack blackish breeding display or markings. Males have elongated, filamentous dorsal- and anal-fin rays (dorsal-fin length 29–31.8 in males vs. 17.4–18.6% SL in females), extending beyond caudal-fin base. Females have a pronounced abdominal concavity and significantly extended (18.9–19.6 vs. 10.2–12.5% SL) pelvic fins compared to males, together forming a “pouch” for pelvic brooding. Due to the less pronounced concavity, males have a deeper body at anal fin origin than females (23.9–24.6 vs. 18.9–22.1% SL), and also a deeper caudal peduncle (11.3–12 vs. 10.4–10.9% SL). The last ray of the pelvic fin is connected to the body by a membrane on half of its length in males, whereas such a membrane is lacking in female O. eversi . Preliminary field and aquarium observations suggest that adult males in breeding mood defend territories, but females form small schools in the natural habitat.
Reproduction. — Oryzias eversi is a “pelvic brooder”, a term coined by Kottelat (1990a) for the lake-dwelling Oryzias (Xenopoecilus) oophorus from Lake Poso in Central Sulawesi, which carry clusters of eggs accommodated in a belly concavity until they hatch. Female O. eversi were observed in the field and in aquaria to carry developing eggs and a preserved female (MZB 20781; compare Fig. 3B View Fig ) carried about 30 eggs of approx. 1.4 mm diameter, with almost fully developed and partially pigmented embryos. Under aquarium conditions (24–25°C), females carry eggs until they hatch after 18–19 days. Aquarium observations showed that the females never deposit eggs on a substrate at all, even if various kinds of spawning substrate are available. Moreover, females change behaviour during brooding and hide among plants during the gestation period. The elongated pelvic fins apparently hold the egg clutch in position in the pronounced belly concavity, strongly resembling other known or suspected pelvic-brooding ricefishes ( Iwamatsu et al., 2007). Like in A. oophorus and O. sarasinorum ( Kottelat, 1990a; Iwamatsu et al., 2008), the eggs do not adhere to each other, and are suspended by attaching filaments to the female’s urogenital pore. After hatching, the attaching filaments protrude out of the female’s urogenital pore for some time ( Fig. 3C View Fig ).
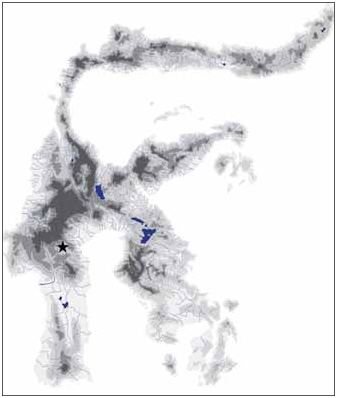
Distribution and habitat. — Oryzias eversi is known to date only from the type locality in Tana Toraja, Central Sulawesi ( Fig. 4 View Fig ). The type locality is an up to approx. 4 m deep karst pond of 30–40 m length and up to 10 m width, used by local people as natural “swimming pool” ( Fig. 5 View Fig ). The water is calm and crystal clear, and had a water temperature of 21.5°C in Sep.2010. The pond has a single in- and outflow and is surrounded by rain forest. Sympatic fish species included native species of Nomorhamphus and introduced Poecilia reticulata . At time of sampling, adult Oryzias eversi were rare but juveniles were rather abundant. Males are solitary, whereas females occur in groups and inhabit the shallow habitat margins characterised by dense vegetation.
Etymology. — The specific name, eversi , honours Hans- Georg Evers who discovered this endemic ricefish while travelling to explore fishes and habitats in Sulawesi.
Comparisons. — Oryzias eversi is clearly distinguished by non-overlapping (33–36) lateral scale counts from Adrianichthys (Lake Poso, including “ Xenopoecilus ” oophorus and “ X.” poptae ;>62), O. orthognathus (Lake Poso,>44), O. sarasinorum (Lake Lindu, 70–75), O. matanensis (Lake Matano,>40) and O. marmoratus (Malili Lakes: Towuti, Mahalona, Lontoa, streams, 31–32) ( Kottelat, 1990b; Parenti, 2008). Lateral scale counts overlap with O. profundicola (Lake Towuti, 32–34), O. bonneorum (36–39), O. nebulosus (32–36), O. nigrimas (34–37), O. hadiatyae (Lake Masapi, 27–31) and O. woworae (Muna Island, 30–33). Oryzias eversi has fewer (17–19) anal-fin rays than O. orthognathus (23–25), O. nigrimas (21–25), O. nebulosus (21–22), O. matanensis (20–25), O. profundicola (26–29), O. marmoratus (20–26), A. oophorus (20–22), A. poptae (24–26(27)), O. sarasinorum (21–23), A. roseni (25–26), and A. kruyti (24–25). Female Oryzias eversi shares with Adrianichthys oophorus , A. poptae , A. roseni , and O. sarasinorum an abdominal concavity between the pelvic fins and anal fin to carry the developing eggs ( Parenti, 2008), a character absent in other ricefish species from Sulawesi. It is however distinguished from Adrianichthys by smaller adult size (largest adult specimen recorded: 38.4 mm SL, vs. adult size of nearly 200 mm SL) and by having only 30–32(33) total vertebrae (vs. 36–37; Parenti, 2008).
Oryzias eversi differs from O. hadiatyae among other characters in having more (½14 vs. ½10–12) transverse rows of lateral scales at dorsal-fin origin. Oryzias eversi lacks the pronounced concavity on the snout (vs. present in O. hadiatyae ), has relatively longer pelvic fins in females (18.9–19.6 vs. 9.8–15.4% SL in O. hadiatyae ), is characterised by rounded dorsal and anal fins with pronounced individual fin rays in males (vs. pointed dorsal fin and rather short anal fin, both without conspicuous individual rays in O. hadiatyae ), and shows clear marginal stripes on the caudal fin (vs. absent in O. hadiatyae ). From O. celebensis , O. eversi is distinguished by having more dorsal fin rays (10–12 vs. 8–10; Parenti, 2008, Herder & Chapuis, 2010), relatively smaller eyes (23.0–35.5 vs. 36.1–45.9% HL; Herder & Chapuis, 2010), conspicuously (vs. slightly) filamentous dorsal- and anal-fin rays in males, and presence (vs. absence) of blackish courtship colouration in adult males. Compared to O. bonneorum , O. eversi has among other characters less principal (i,4/5,i vs. i,5/6,i) and more procurrent (dorsal 6–7, ventral 6–8 vs. dorsal 5, ventral 5–6) caudal fin rays, less rays in dorsal- (10–12 vs. 12–13), anal- (17–18(19) vs. 19–20) and pectoral fin (10 vs. 11–12), and no pronounced abdominal concavity. Oryzias eversi is distinguished from O. woworae by its larger adult size (up to 38.4 mm vs. 28.0 mm SL; Parenti & Hadiaty, 2010), presence (vs. absence) of an abdominal concavity, and by features of adult male breeding colour pattern, most conspicuously the absence (vs. presence) of steel blue body colouration and absence (vs. presence) of red colouration in ventral surface of head and body anterior to pelvic fins, dorsal portion of pectoral fins, bases of dorsal and anal fin, and margins of caudal fin in both sexes.
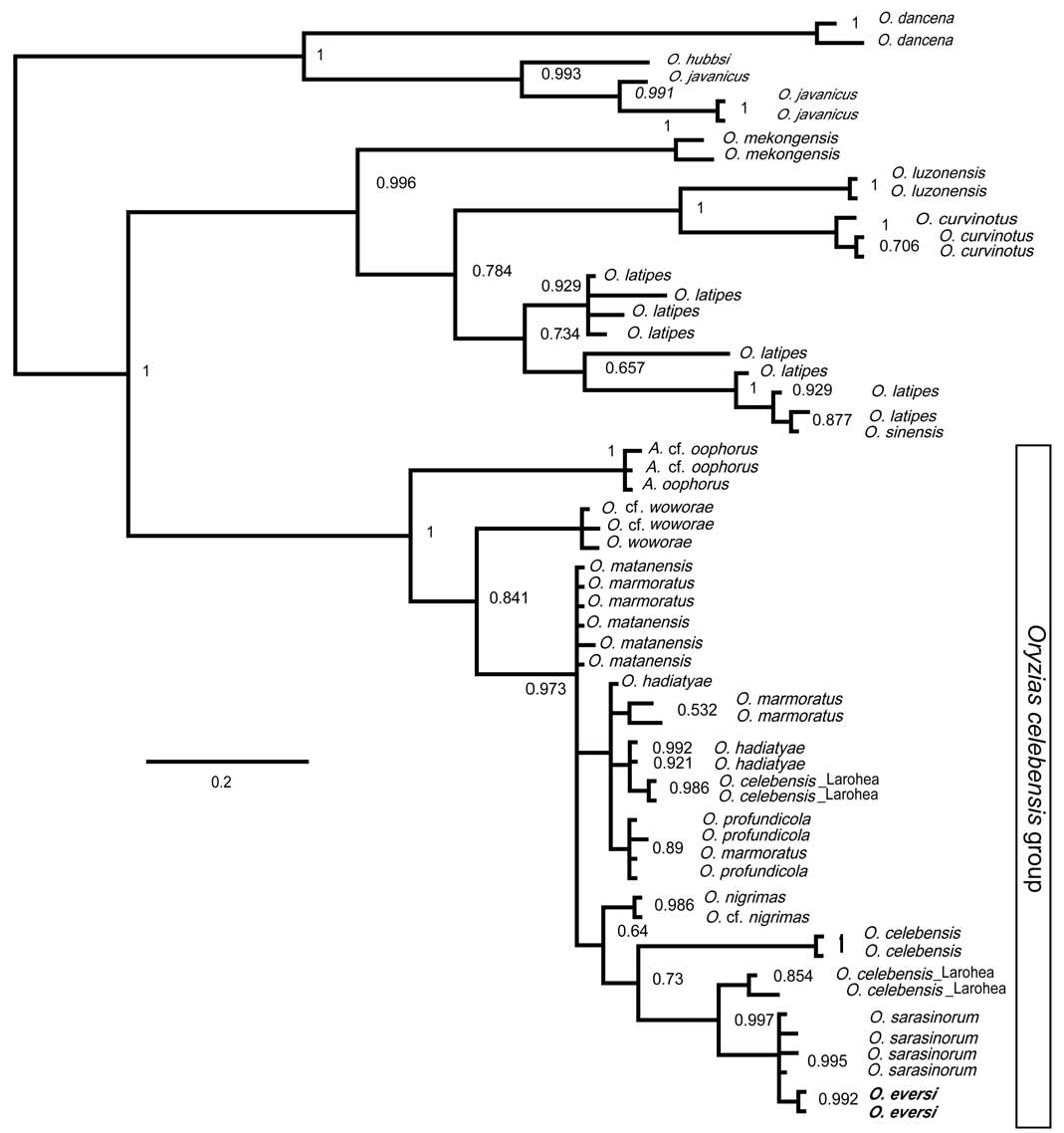
Phylogenetic relationships. — The model that best reflected the evolutionary divergence of the 16s rDNA sequences (SYM+I+G) was selected by MrModeltest 2.3 and was used to infer the most likely Bayesian phylogeny ( Fig. 6 View Fig ). The resulting Bayesian phylogeny is largely congruent with the consensus tree of Takehana et al. (2005) in all well supported branches, including monophyly of all known Oryzias from Sulawesi (the “ Oryzias celebensis species group”, distinguished also by karyological characters; Takehana et al., 2005). In agreement with shared pelvic brooding ecology and associated morphological characters, the present analyses clearly support a sister group relationship between O. eversi and O. sarasinorum from Lake Lindu in Central Sulawesi. These two pelvic brooders are more closely related to a number of egg-depositing species (i.e., Oryzias matanensis , O. marmoratus , O. profundicola , and O. hadiatyae endemic to the Mallili lakes, O. nigrimas from Lake Poso, and the riverine species O. celebensis , O. woworae and the hitherto undescribed O. cf. woworae [Parenti et al., in prep.]) than to the only other pelvic brooder ( Adrianichthys oophorus ) included in our analyses. The phylogenetic relationships among the egg-depositing taxa is not fully resolved and warrants further study. The Malili lakes endemics ( Oryzias matanensis , O. marmoratus , O. profundicola , O. hadiatyae ) share closely related haplotypes across species, including some individuals of O. celebensis from Larohea. The latter is a recently discovered ricefish population from a drainage west of Lake Matano in Central Sulawesi fitting the morphological concept of O. celebensis ; this species was known until recently only from the south-western arm of the island (Herder & Chapuis, 2010). Conspicuously however, some of the O. celebensis from Larohea carry very distinct mitochondrial haplotypes most closely related to O. sarasinorum from Lake Lindu and to O. eversi .
| MZB |
Museum Zoologicum Bogoriense |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.