Oophaga vicentei (Jungfer, Weygoldt & Juraske, 1996)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5175.3.8 |
|
DOI |
https://doi.org/10.5281/zenodo.7010108 |
|
persistent identifier |
https://treatment.plazi.org/id/0B1B879E-FFA3-FFD6-BFB2-F88B06CA2A3B |
|
treatment provided by |
Plazi |
|
scientific name |
Oophaga vicentei |
| status |
|
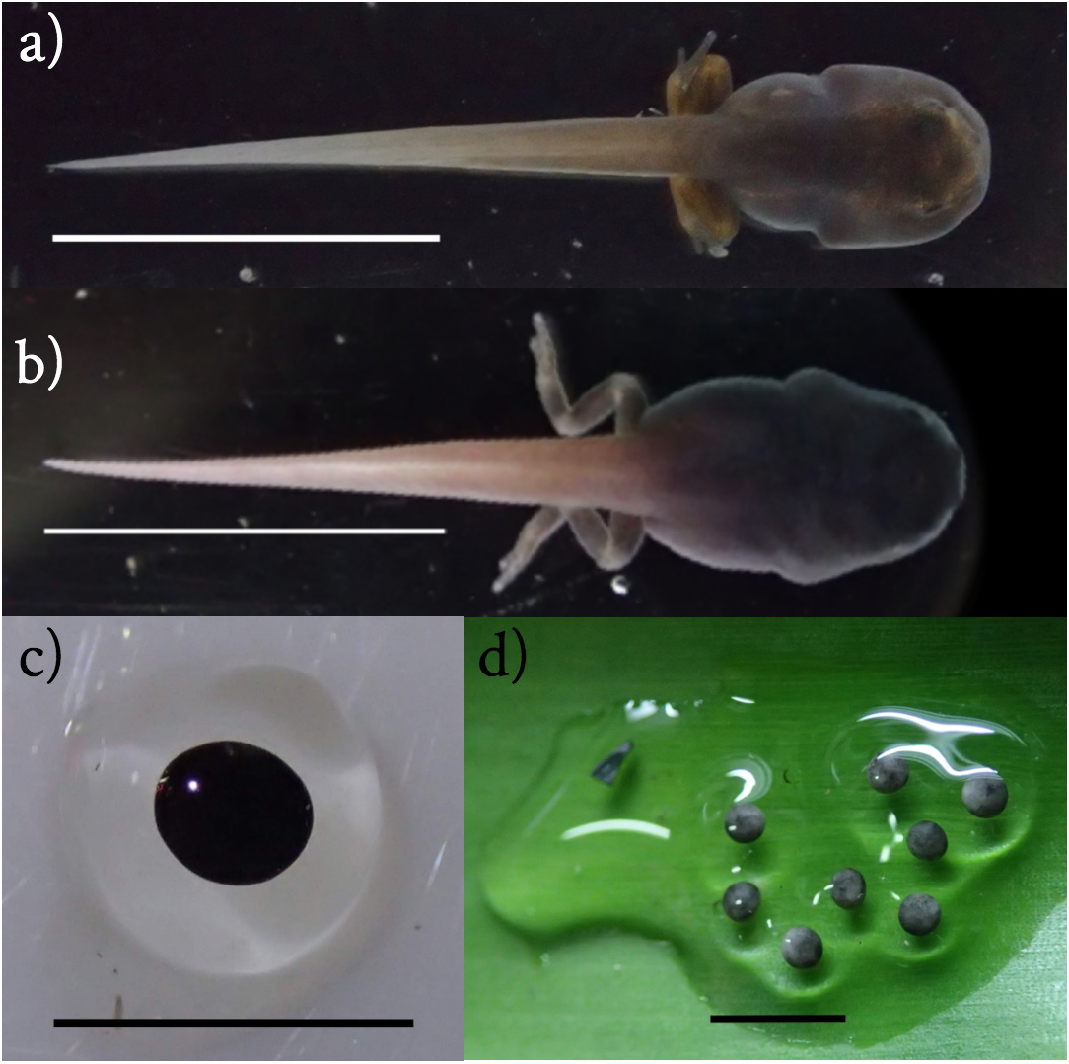
Body length and total length measurements of O. vicentei tadpoles are summarized in Table 1 View TABLE 1 . The body is ovate and slightly tapered at the anterior end ( Fig. 1B View FIGURE 1 ). Eyes are located dorsally and oriented anterolaterally. Nares are round without projection in the inner margin of the nasal rim and are located dorsally. The vent tube is median, and the spiracle is sinistral. The oral disc is round and not indented, with enlarged oral papillae surrounding the posterior labium and the lateral margins of the anterior labium. LTRF is 1[1]/0 in all but one specimen in the sample, a Stage 41 tadpole that also has a posterior labial tooth row. We can not rule out the possibility that decomposition may have played a role in the number of specimens observed to lack a posterior labial tooth row. The anterior labial teeth are scarce and large, and the jaw sheaths are thick and serrate, the upper sheath U-shaped. The oral disc is oriented anteroventrally. In ethanol, O. vicentei specimens are uniformly gray in colour from above and below and translucent around the margins of the tail. A differentiated short gut in the abdominal area can be noticed. Live specimens are dark grey to black in colour, with darker speckling on the body and tail, and translucent along the margins of the tail ( Fig. 2B View FIGURE 2 ).
Thirteen O. vicentei eggs from 5 different breeding pairs were examined in detail. In our sample, there was 1 clutch of 4 eggs, 2 clutches of 3 eggs, 1 clutch of 2 eggs (only 1 egg measured), and 2 clutches of 1 egg, but there may be anywhere between 1 and 12 eggs per clutch for this species, based on past observations. The clutches formed a cohesive transparent mass of eggs ( Fig. 2D View FIGURE 2 ). The eggs (n = 13, embryo with jelly coat) measured approximately 3 mm in diameter. The embryos at Stages 0–3 had varying amounts of light grey at the vegetal pole, and dark grey at the animal pole, and had an average diameter of 1.6 mm (n = 13, SD = 0.10 mm).
Andinobates altobuyensis , A. claudiae , A. fulguritus , A. geminisae and A. minutus tadpoles have a complete row of oral papillae along the posterior edge of the oral disc. This feature distinguishes them from other species described, all which have a large medial gap interrupting the posterior row of oral papillae ( Bernal et al. 2007; Brown et al. 2011; DuarteMarín et al. 2020; Myers & Daly 1976, 1980; Ruiz-Carranza & Ramírez-Pinilla 1992; Silverstone 1975). Myers & Daly (1980) first proposed the medial gap in the posterior oral papillae as a synapomorphy, joining A. abditus , A. bombetes , A. daleswansoni , A. opisthomelas , A. tolimensis and A. virolinensis in the A. bombetes group ( Brown et al. 2011; DuarteMarín et al. 2020). Current distribution of this character is consistent with molecular phylogenetic hypotheses (e.g., Amézquita et al. 2013; Márquez et al. 2017). Nonetheless, the oral papillae along the posterior margin of the oral disc of A. supata tadpoles require further examination, since no gap has been reported for this species of the A. bombetes group ( Chaves-Portilla et al. 2021).
Tadpoles from the genus Oophaga can be distinguished from tadpoles from other dendrobatid genera by their tapered egg-shaped body, reduced tooth rows (maximum one anterior and one posterior row) and the large size of the scarce oral papillae lining the entire posterior labium and the lateral regions of the anterior labium. However, we could not distinguish between described tadpoles of the genus Oophaga based on morphology alone. Oophaga vicentei tadpoles are indistinguishable from O. arborea , O. granulifera , O. histrionica , O. pumilio and O. speciosa for most observed traits ( Jungfer 1985; Myers et al. 1984; Savage 1968; Silverstone 1975; Starrett 1960; van Wijngaarden & Bolaños 1992). Oophaga vicentei tadpoles differ from the only O. speciosa tadpole specimen described that lacks the anterior tooth row ( Jungfer 1985).
Oophaga spp. tadpoles exhibit a reduced oral morphology that is typical of egg-eating dendrobatid tadpoles ( van Wijngaarden & Bolaños 1992; Caldwell & De Araújo 1998). Furthermore, Oophaga spp. tadpoles share oral morphological features (i.e., an antero-ventrally positioned mouth, reduced tooth rows and tooth number, large jaw sheaths) that are considered to be adaptations to an egg-based diet in other groups of anuran larvae ( Kishimoto & Hayashi 2017; Kuramoto & Wang 1987; Wassersug et al. 1981), and could be well-conserved in the genus Oophaga because they hold a similar adaptive significance.
Andinobates minutus eggs taken from the ovary of a dissected female were 3 to 4 mm in diameter ( Silverstone 1975), but these had not likely acquired their full size yet. Information on eggs from other congeneric species is missing, except for a brief note of “brown eggs”, of unknown stage or state of preservation, from A. daleswansoni , A. dorisswansonae and A. tolimensis ( Bernal et al. 2007; Rueda-Almonacid et al. 2006). Our observations on the clutch size of O. vicentei eggs are consistent with reports from Jungfer et al. (1996), who indicated that a female laid 2- 6 eggs after mating.
All protocols for this study were approved by the institutional animal care and use committee (IACUC, protocol #2016-0311-2019-A6) and the University animal care committee (UACC, protocol # 1237B-17). This project would not have been possible if it were not for the efforts of the staff, interns, and volunteers of the Panama Amphibian Rescue and Conservation (PARC) project (J. Guerrel, N. Fairchild, E. Lassiter, N. Cabezón, L. Cheucarama, J. Warren, G. Ureña, O. Ariel Garcés, and V. Franco), who work tirelessly to provide the best possible care for Panama’s most vulnerable captive amphibian populations, and who were always willing to lend a hand with the tasks related to this paper. We especially thank E. Lassiter for his help photographing specimens, collecting data, and assisting KH with a variety of other tasks related to this project, as well as J. Guerrel for staying after hours to help plan data collection and animal care protocols that would facilitate this research. We also want to thank Dr. A. Mooers for his endless support and encouragement over the entire course of this project and for helping to edit this paper, Dr. Brian Gratwicke for his financial support for this project and Dr. Wendy Palen for making herself available to discuss ideas with KH as she carried out her work in Panama. RI was supported by the PARC project, and the Sistema Nacional de Investigación of the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT). The ex situ management of captive populations and breeding efforts on these two species are largely subsidized by Minera Panamá-Cobre Panamá of First Quantum Minerals Ltd.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.