Nemesia arboricola Pocock, 1903
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.806.1705 |
|
publication LSID |
lsid:zoobank.org:pub:4991BA30-038F-4B86-983B-E4E738C56759 |
|
DOI |
https://doi.org/10.5281/zenodo.6385311 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA934D-0A66-D14A-9432-3EBDFAA8F9E4 |
|
treatment provided by |
Felipe |
|
scientific name |
Nemesia arboricola Pocock, 1903 |
| status |
|
Nemesia arboricola Pocock, 1903 View in CoL
Figs 1–4 View Figs 1–8 , 9–14 View Figs 9–20 , 23, 25 View Figs 21–26 , 27–53 View Figs 27–35 View Figs 36–44 View Figs 45–53
Nemesia arboricola Pocock, 1903: 225–226 View in CoL (♀).
Nemesia arboricola View in CoL – Baldacchino et al. 1993: 40. — Kritscher 1994: 49–57, figs 1–18 (♀); 1996: 121 (♀). — Dandria 2001: 103–107, fig. 1 pl. 1 (tree nest). — Le Peru 2011: 77. — Decae 2012: 25, fig. 2 (species groups).
New diagnosis
Female
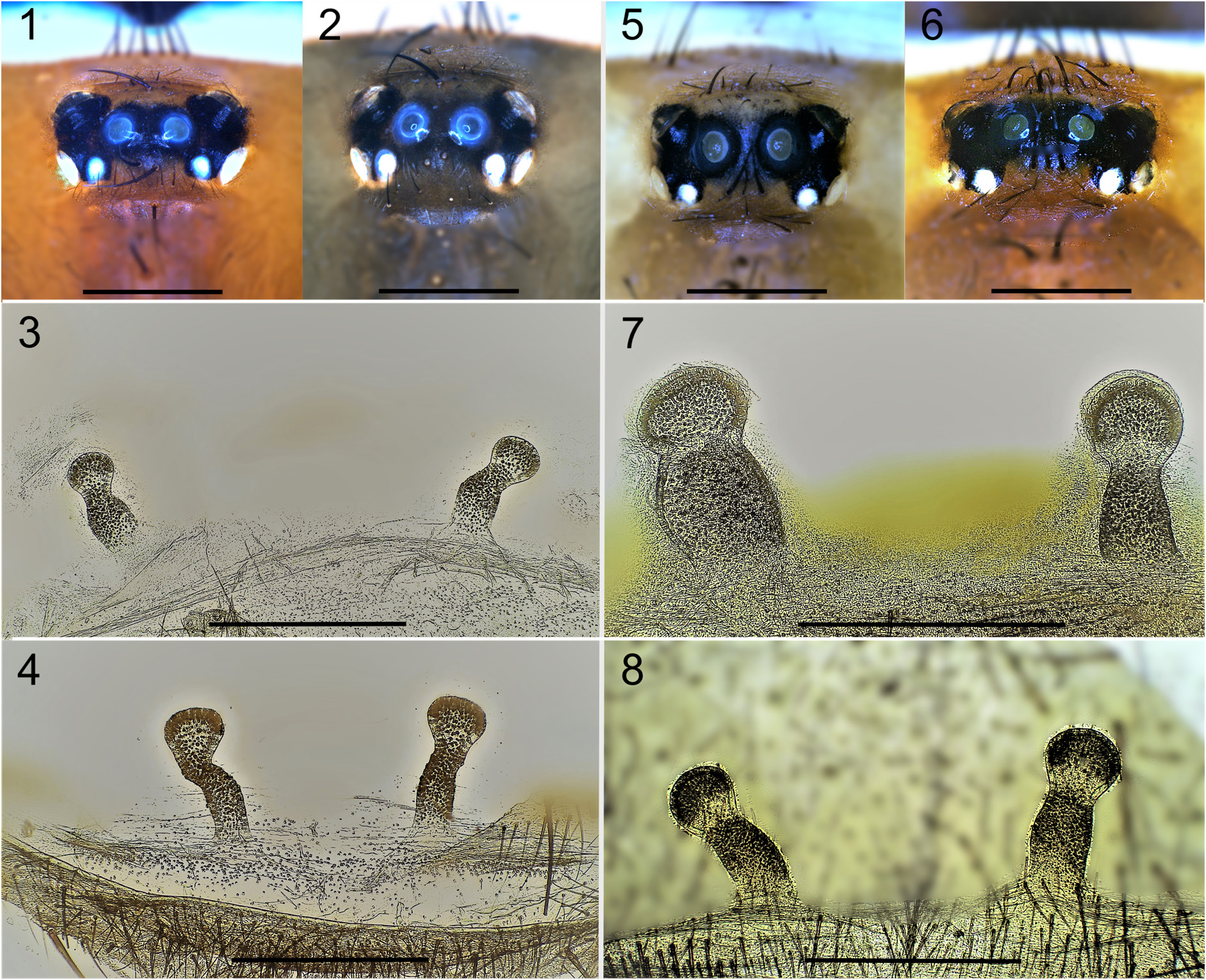
Nemesia arboricola from Malta is difficult to distinguish from N. macrocephala from nearby Sicily ( Kritscher 1994). Females of the two species are among the largest Nemesia species known (fully grown females in both species range from 26 to 28 mm in total body length; the average in Nemesia is around 17.5 mm), and show only slight differences in their sexual and somatic morphology. Kritscher (1994) used the following three diagnostic characters to distinguish females of N. arboricola from those of N. macrocephala : (1) a different distance between the ALE and PLE, (2) a different shape of the spermathecal receptacles, (3) the presence or absence of labial cuspules. Pocock (1903) also reports the configuration of the eyes and the presence of labial cuspules as distinctive for N. arboricola . We found that, in general, the configuration of eyes and in particular the distance between the ALE and PLE ( Figs 1–2 and 5–6 View Figs 1–8 ) are unsuitable to distinguish N. arboricola from N. macrocephala on grounds of overlapping and highly variable measurements (dis. ALE–PLE av. 0.46, sd. 0.13 and av. 0.52, sd. 0.18, respectively). Although the shape of the receptacles appears to differ between the two species ( Figs 3–4 and 7–8 View Figs 1–8 ) it is difficult to quantify these differences. We therefore regard this character as only marginally reliable as diagnostic. We confirm Kritscher’s third diagnostic character (also noted in Pocock 1903) as truly diagnostic and agree that the presence of labial cuspules in females is distinctive for N. arboricola ( Figs 9–10 View Figs 9–20 cf. Figs 15–16 View Figs 9–20 ). Furthermore, we found the dark coloured and speckled opisthosoma in N. arboricola ( Figs 11–12 View Figs 9–20 ) versus the lighter coloured opisthosoma with chevron stripes in N. macrocephala ( Figs 17–18 View Figs 9–20 ), and the thicker, slightly swollen, PMS ( Figs 13–14 View Figs 9–20 cf. Figs 19–20 View Figs 9–20 ) in N. arboricola as diagnostic characters to distinguish females of the two species.
Male
Because the male of N. macrocephala remains unknown, no characters that might distinguish this species from N. arboricola can presently be given. The male of N. arboricola however differs from nearly all Nemesia species, for which the relevant information is available, by the absence of the PTR (see section ‘terminology’ above). The only other species known in which the PTR is missing is N. simoni O. Pickard- Cambridge, 1874 from southwestern France and northern Spain. To illustrate the presence and/or absence of PTR in different species of Nemesia species, Figs 21–24 View Figs 21–26 show the dorso-distal male palps of four different Nemesia species collected from geographically widely separated locations in the Nemesia distribution range ( N. bacelarae Decae, Cardoso & Selden, 2007 from Portugal ( Fig. 21 View Figs 21–26 ), N. cellicola Audouin, 1826 from the Middle East ( Fig. 22 View Figs 21–26 ), N. simoni from southern France ( Fig. 23 View Figs 21–26 ) and N. arboricola ( Fig. 24 View Figs 21–26 ) from Malta). Note that species from opposite far ends of the distribution range and a new species from centrally located Malta ( Fig. 56 View Figs 54–63 ) feature a pronounced PTR only absent in N. arboricola and N. simoni ( Figs 23–26 View Figs 21–26 ). Nemesia arboricola males can be distinguished from those of N. simoni by their generally larger size (TBL » 13, n = 2 vs TBL » 11, n = 7) and on several aspects of its sexual and somatic morphology, its burrow structure and its distribution (compare the male description below with information given on N. simoni in Moggridge 1873). Figs 25–26 View Figs 21–26 show distinctive differences in male palps (shape of tibia) and palpal organs of N. arboricola and N. simoni .
Holotype
In collection BMNH, London, not examined.See Kritscher(1994) for a detailed description and photographs of the specimen (in very poor and fragile condition), and for conformation that N. arboricola is the common and widely distributed cork-door building Nemesia species found in the Maltese Archipelago.
Material examined
MALTA • 1 ♂; Comino ; 36.02° N, 14.33° E; 23 Mar. 1975; P.J. Schembri leg.; (no. TD.4); NHMR GoogleMaps • 1 ♀; Wied Incita ; 35.88° N, 14.43° E; 25 Oct. 1980; P.J. Schembri leg.; (no. TD.1); NHMR GoogleMaps • 1 ♀; Siggiewi, Buskett ; 35.86° N, 14.39° E; 7 Sep. 1976; P.J. Schembri leg.; (no. TD.2); NHMR GoogleMaps • 1 ♀; St. Paul’s Island ; 35.96° N, 14.40° E; 20 Apr.1975; P.J. Schembri leg.; (no. TD.3); NHMR GoogleMaps • 1 ♂; Gudja; 35.85° N, 14.50° E; 7 Nov. 2020; T. Cassar leg.; (no. TC-011); NHMW GoogleMaps • 1 ♀; Zebbug; 35.87° N, 14.44° E, 27 Aug. 2020; T. Cassar leg.; (no. TC.014 terrestrial); NHMW GoogleMaps • 1 ♀; Buskett ; 35.86° N, 14.39° E; 5 Sep. 2020; T. Cassar leg.; (no. TC.015, arboreal); NHMR GoogleMaps • 1 ♀; Valetta, Imsida; 35.89° N, 14.48° E; 20 Dec. 2017; (no. TC.016 arboreal); NHMR GoogleMaps • 1 ♀; Comino ; 36.02° N, 14.33° E; 11 Sep. 2020; T. Cassar leg.; (no. TC.017 terrestrial); NHMR GoogleMaps .
Description
Male reference specimen (no. TD.4, NHMR)
PRESERVATION AND CONDITION. Specimen 45 years preserved in 70% ethanol, generally in good condition ( Fig. 27 View Figs 27–35 ), right bulb removed for detail study ( Figs 31–35 View Figs 27–35 ).
GENERAL COLORATION. Carapace uniform dark brown, chelicerae slightly darker than carapace, abdomen dorsal dark grey with light grey speckles in two parallel rows in the cardiac region ( Fig. 27 View Figs 27–35 ), abdomen ventral light yellowish brown, palps uniform dark brown, legs dark brown proximally grading to light brown distally, sternum uniform yellowish brown, labium dark brown.
CARAPACE. Longer than wide (CW/CL 0.8), with light grey pubescence cover (partly lost), bristles concentrated along the margins and posterior on the thoracic part, cephalic part slightly elevated, thoracic part bulging slightly up from the fovea before sloping down to the posterior margin ( Fig. 28 View Figs 27–35 ).
EYES. Ocular-tubercle ( Fig. 28 View Figs 27–35 ) dome-shaped, clypeus narrow sloping down from eyes, eye-group rectangular, twice as wide as long (EL/PR 0.51), AME about their diameter apart (dis.AME/dia.AME 0.94), dis.ALE–PLE <dia.ALE (ALE–PLE/ALE 0.60).
CHELICERAE. Rastellum with few very strong apical teeth, teeth of diminishing strength and size along distal prolateral margin of chelicerae, single, prolateral row of furrow teeth, fang proximally bent, ventral serrated ridge.
VENTRAL PROSOMA. Maxillae rounded distal lobe, cuspules absent; labium: wider than long (LW/LL 1.6) distally truncated, cuspules absent, labial furrow wide, with two distinct semi-circular sigilla; sternum: longer than wide (SW/SL 0.8) three pairs oval sigilla, even cover of bristles.
PALPS. Cymbium with distal group of spines, tibia proximally lightly inflated (TibW/PTib 0.4),PTR absent ( Figs 23, 25 View Figs 21–26 ), patella spineless, femur with few dorso-distal spines, longer than tibia (PFem/PTib 1.3); palpal organ: proximal bulbous part simple pyriform, embolus strong, stiff, curved in ventral and dorsal views ( Figs 31, 33 View Figs 27–35 ), straight and distally narrowing in prolateral and retrolateral views ( Figs 32, 34 View Figs 27–35 ), embolus tip blunt, and slightly flattened or slightly scooped ( Fig. 35 View Figs 27–35 ).
LEGS. All tarsi light coloured with fine ventral scopulae, all femora cylindrical with few spiny bristles dorsally, metatarsus I curved, CF with fine, short bristles ( Fig. 29 View Figs 27–35 ), ventro-apical spines and one central prolateral spine, tibia I distally widened, TA distinct, TS distally slightly sigmoid ( Fig. 29 View Figs 27–35 ), central row of three prolateral spines, patella I with a single prolateral spine, metatarsus and tibia II cylindrical with few spines, patella II with two prolateral spines, metatarsus and tibia III as II, patella III with single prolateral spine ( Fig. 30 View Figs 27–35 ), metatarsus IV> femur IV> tibia IV (Fem4/Met4 = 0.97, Tib4/Met4 = 0.83), patella IV 0–1 retrolateral patellar spine, PTC all tarsi double combs of teeth, leg formula: 4123.
OPISTHOSOMA. Ovoid, anterior narrowing, covered with bristles, PMS small, close together and less swollen than in females, PLS short, thick, proximal segment longer than medial + distal segment, spigots restricted to apical spigot field.
MEASUREMENTS. TBL = 13.0; CL = 6.9; CW = 5.7; CP = 3.8; AR = 1.07; PR = 1.07; EL = 0.55; dia.ALE = 0.25; dia.PLE 0.21; dia.AME = 0.18; dia.PME = 0.17; dis.AME–AME = 0.17; dis.ALE–PLE = 0.15; SL = 3.6; SW = 2.9; LL = 0.6; LW = 1.0; Palp = 9.5 (1.3 + 2.8 + 1.8 + 3.6); Leg I = 20.3 (2.9 + 4.3 + 4.1 + 3.1 + 5.9); Leg II = 19.6 (2.6 + 4.6 + 4.2 + 2.9 + 5.3); Leg III = 19.5 (2.7 + 5.0 + 3.9 + 2.6 + 5,3); Leg IV = 25.0 (3.2 + 6.6 + 5.5 + 3.2 + 6.5); BuL = 2.11; BuW = 0.79; EmL = 1.06.
VARIATION MALES (n = 2). TBL = 12.8, 13.0; CL = 5.8, 6.9; CW = 5.0, 5.7; CP = 3.5, 3.8; AR = 1.07, 1.11; PR = 1.07, 1.08; EL = 0.55, 0.56; dia.ALE = 0.25, 0.27; dia.PLE = 0.21, 0.22; dia.AME = 0.18; dia. PME = 0.15, 0.17; dis.AME–AME = 0.17, 0.20; dis.ALE–PLE = 0.10, 0.15; SL = 3.1, 3.6; SW = 2.5, 2.9; LL = 0.5, 0.6; LW = 0.9, 1.0; Palp = 8.6, 9.4; Leg I = 18.0, 20.3; Leg II = 17.3, 19.7; Leg III = 16.9, 19.4; Leg IV = 22.5, 24.9; Bul = 1.88, 2.11; BuW = 0.71, 0.79; EmL = 0.78, 1.06.
Notes on female
Females of N. arboricola have been described in proper detail by Pocock (1903) and Kritscher (1994). Here we compare specimens collected from tree-nests with specimens collected from terrestrial burrows. Figures 36–42 View Figs 36–44 , illustrate the variation in general appearance of the specimens in our study sample. Although individual variation in size, colour patterns and shades are evident, no qualitative morphological differences were found that would distinguish tree dwelling spiders from ground dwelling spiders at the species level. Moreover, all specimens in our sample closely fit the descriptions given by Pocock (1903) and Kritscher (1994). On these grounds we feel confident to state that arboreal and terrestrial specimens studied here are conspecific members of N. arboricola Pocock (1903) . Figures 43–44 View Figs 36–44 , show characters common to all N. arboricola specimens (terrestrial and arboreal) studied, that distinguish N. arboricola from its supposed sister species, N. macrocephala .
Mesurements, variation females (n = 7) TBL = 21.1–26.9 (av. 23.2, sd. 2.5); CL = 7.8–9.1 (av. 8.3, sd. 0.6); CW = 6.4–7.5 (av. 6.8, sd. 0.4); CP = 4.8–5.6 (av. 5.1, sd. 0.3); AR = 1.33–1.51 (av. 1.41, sd. 0.07); PR = 1.29–1.51 (av. 1.38, sd. 0.09); EL = 0.53–0.77 (av. 0.71, sd. 0.09); dia.ALE = 0.26–0.41 (av. 0.35, sd. 0.05); dia.PLE = 0.21–0.34 (av. 0.29, sd. 0.04); dia.AME = 0.13–0.20 (av. 0.18, sd. 0.03); dia.PME = 0.14–0.23 (av. 0.18, sd. 0.03); dis.AME–AME = 0.20–0.34 (av. 0.26, sd. 0.05); dis.ALE– PLE = 0.10–20 (av. 0.16, sd. 0.04); SL = 3.8–5.2 (av. 4.7, sd. 0.5); SW = 3.6–4.1 (av. 3.8, sd. 0.2); LL = 0.9–1.1 (av. 1.0, sd. 0.1); LW = 1.4–1.6 (av. 1.5, sd. 0.1); Palp = 11.9–13.5 (av. 12.5, sd. 0.8); Leg I = 16.1–18.7 (av. 17.1, sd. 1.0); Leg II = 14.6–16.9 (av. 15.4, sd. 0.8); Leg III = 14.5–16.9 (av. 15.5, sd. 0.9); Leg IV = 20.1–23.4 (av. 21.5, sd. 1.2).
Field observations
Nemesia arboricola constructs a simple tube-shaped burrow, capped with a sturdy lid (trapdoor) fitting neatly into the shaft ( Figs 46, 51 View Figs 45–53 ). Apparently older nests have been found with a layer of moss growing on the door surface ( Fig. 45 View Figs 45–53 ). The burrows are constructed in natural settings such as in tree trunks, between rocks in rubble walls and sloping ground ( Figs 45–53 View Figs 45–53 ). Tree-nests have been found between 150 cm and 8 cm above the ground surface in the trunks of ornamental palms ( Phoenix canariensis and P. dactylifera ), carob trees ( Ceratonia siliqua ) and olive trees ( Olea europaea ). In such tree-nests, spiders make use of pre-existing hollows and holes in the tree trunks into which they can snugly fit, or make use of hollows which are filled with woody debris and soil into which they can easily burrow. Burrows have also been found, at similar heights above the ground surface, in old rubble walls which hold back soil at field margins; here the spiders burrow into the clayey soil which fills the cracks between the individual stones. In both tree-nests and wall-nests, the burrow lids are flush with the vertical surface and dorsally hinged so that the trapdoor opens vertically upwards, with the shaft directed into the trunk/ wall perpendicular to the vertical axis. Nemesia arboricola also nests directly in the ground, but always it seems under one of the following conditions: the ground must be steeply sloping or, if the soil is flat, located immediately beneath an overhanging structure such as large rocks or a rubble wall ( Figs 52–53 View Figs 45–53 ). No burrows have been found on level ground in full exposure (‘in plain sight’ as it were). Tree-nests and wall-nests vary from 4.5 cm to 9.5 cm in depth for mature individuals, with burrow width varying according to the size (maturity) of the individual. Ground-nests in deep soil have, on average, deeper shafts than tree or wall-nests. In all cases, the burrow shaft is relatively simple in construction; it has entire, thick lining of silk with no internal doors, plugs or other features; and it is unbranched.
The resident spiders themselves respond to disturbance by holding the hinged door very tightly; if the door is prised open using forceps (which takes considerable force) the spider may either retreat immediately head-up to the bottom of the shaft, or else repeatedly attempt to close the door while striking the forceps aggressively with its chelicerae. Recently hatched juveniles are retained within the mother’s burrow, from which they disperse and apparently establish themselves in close proximity, as N. arboricola populations are always localized and often quite dense. Moulted exoskeletons are discarded from the burrow and may be observed outside near the burrows if they are sheltered from wind. During the aestivation period, the door to the burrow is sealed shut from the inside, but the door still remains exposed as under normal circumstances (i.e., the burrow lid is not concealed with soil during periods of inactivity). Most mature males emerge from their burrows and wander between the months of November and March, though a few individuals may appear outside this timeframe.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Mygalomorphae |
|
Family |
|
|
Genus |
Nemesia arboricola Pocock, 1903
| Cassar, Thomas, Mifsud, David & Decae, Arthur E. 2022 |
Nemesia arboricola
| Decae A. E. 2012: 25 |
| Le Peru B. 2011: 77 |
| Dandria D. 2001: 103 |
| Kritscher E. 1994: 49 |
| Baldacchino A. E. & Dandria D. & Lanfranco E. & Schembri P. J. 1993: 40 |
Nemesia arboricola
| Pocock R. I. 1903: 226 |