Liphistius indra, Peter J. Schwendinger, 2017
|
publication ID |
https://doi.org/ 10.5281/zenodo.893555 |
|
DOI |
https://doi.org/10.5281/zenodo.6042376 |
|
persistent identifier |
https://treatment.plazi.org/id/4C30A452-FFFB-FFD4-BB2F-FB753979FC9D |
|
treatment provided by |
Plazi |
|
scientific name |
Liphistius indra |
| status |
sp. nov. |
Liphistius indra View in CoL sp. nov.
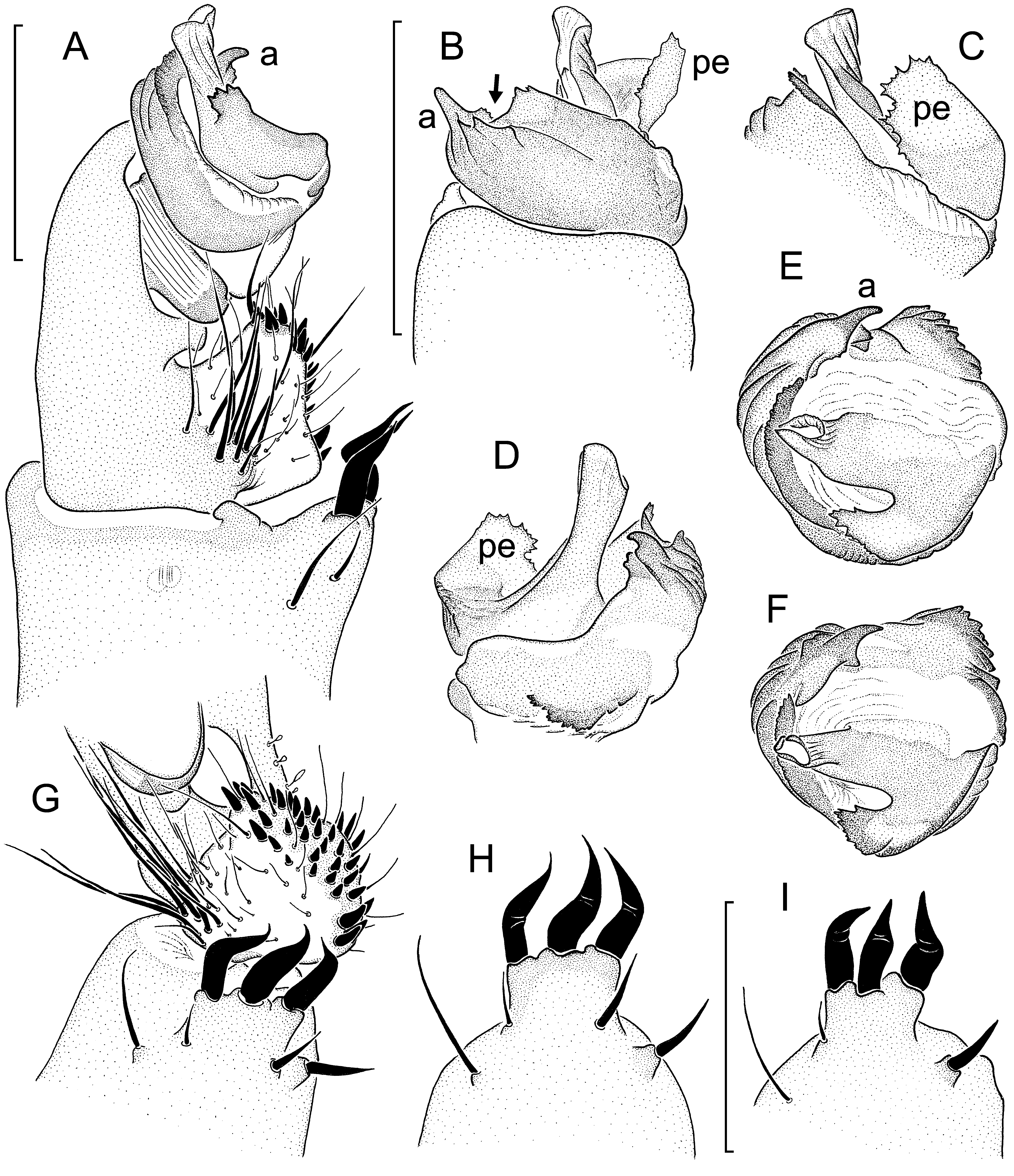
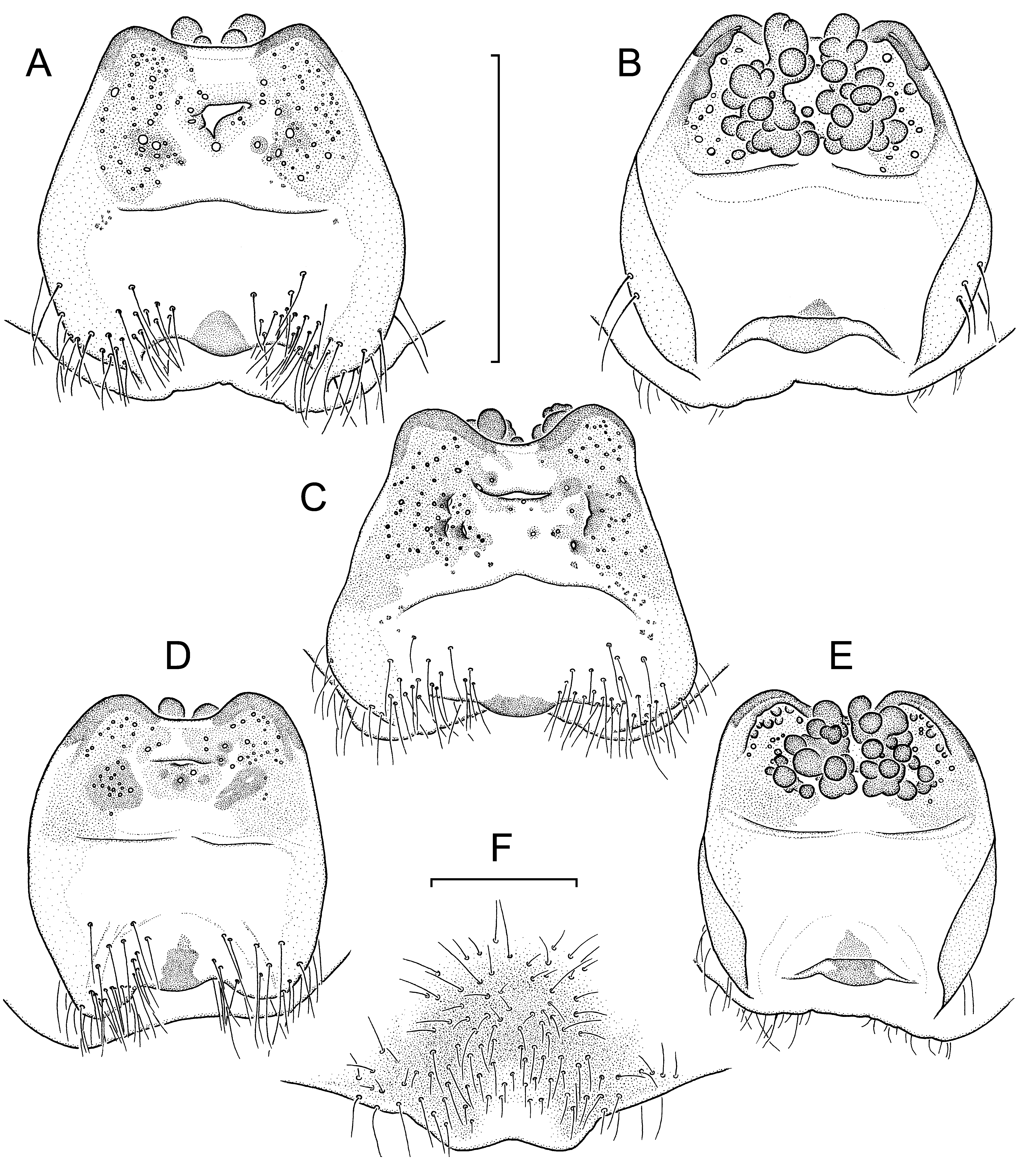
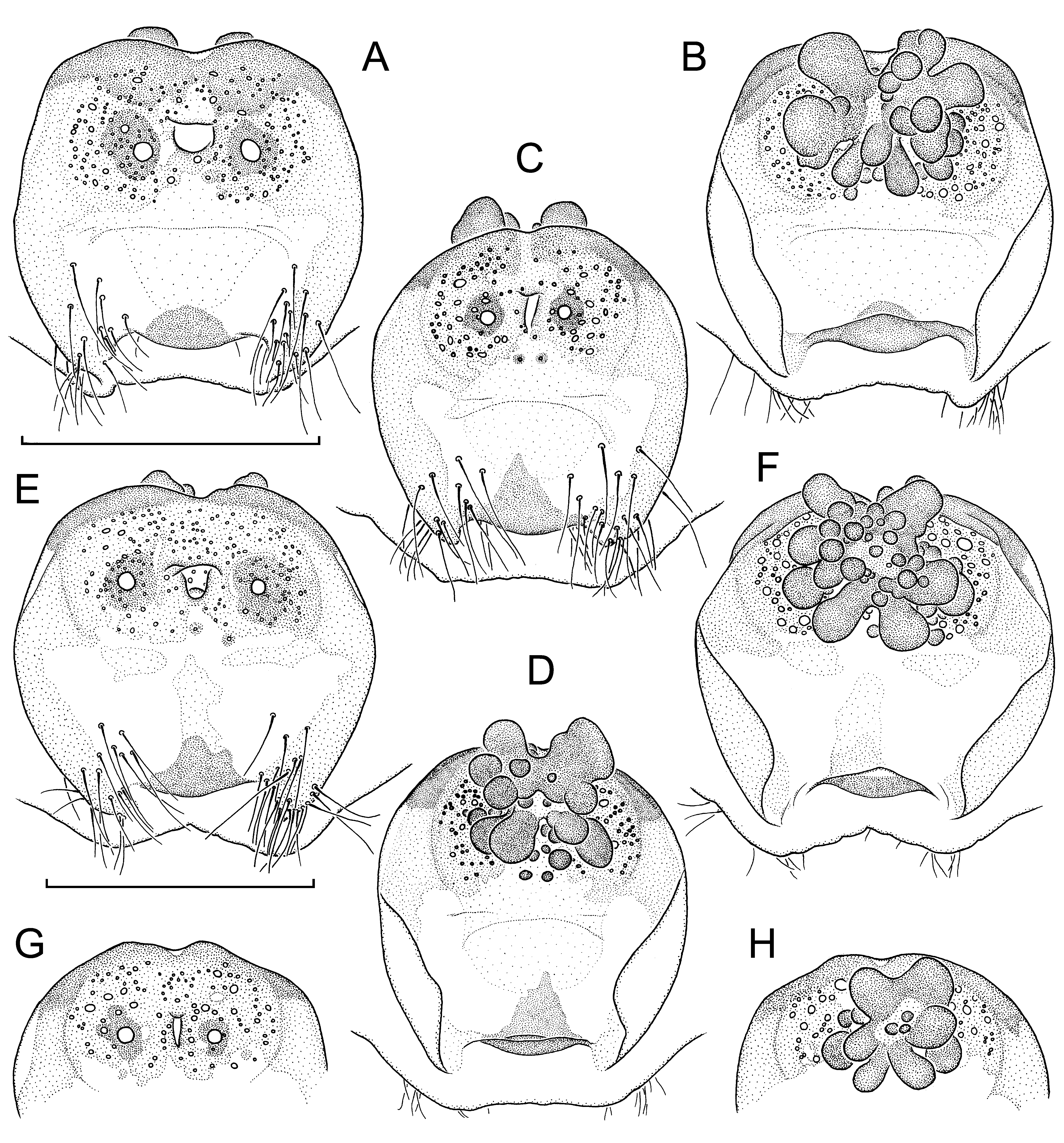
Figs 2B View Fig. 2 , 17-18 View Fig. 17 View Fig. 18
Types: MHNG; male holotype (matured 27.IX.2011), 8 male (matured 24.VIII., 7.IX., 10.IX., 17.IX., 25.IX., 26.X.2011, 30.IX., 4.X.2012) and 13 female paratypes (including allotype which did not moult); Thailand, Pattani Province, about 20 km NW of Yala, Sankalakhierie Mountains , 6°39’09”N, 101°05’55”E, 200 m; 12.VII.2011; leg. P.J. Schwendinger. GoogleMaps – MHNG, SMF; 1 male (matured 17.XI.2000) and 5 female paratypes; same locality, 260 m; 22.X.1999; leg. P.J. Schwendinger. GoogleMaps
Etymology: The species epithet refers to one of the two old names of the type locality: Indragiri (= Indra’s mountain) and Bukit Besar (= big mountain) ( Skeat, 1953: 21). Indra (“Phra In” in Thai mythology) is the king of the Vedic gods. Name in apposition.
Diagnosis: Medium-sized, light brown-coloured species in both sexes. Very similar to L. linang sp. nov., distinguished by males with a ventral scopula on tarsus IV; long bristle retroventrally below tibial apophysis much stronger, resembling a megaspine ( Fig. 17A View Fig. 17 , I-K cf. Fig. 15A View Fig. 15 , G-I); paracymbium distinctly shorter, more globular ( Fig. 17A, I View Fig. 17 cf. Fig. 15A, G View Fig. 15 ); distal edge of contrategulum without dentate gap, ending in a beaklike rather than a hook-like dorsal apex ( Fig. 17B View Fig. 17 , G-H cf. Fig. 15B View Fig. 15 , E-F). Females distinguished from those of L. linang sp. nov. by anterior margin of poreplate less deeply and less widely invaginated; very weak pigmentation (in contrast to none) in anterior part of genital atrium (corresponding to reduced anterior part of posterior stalk); CDO semicircular or longitudinally slit-like instead of transversally slit-like, flanked by an enlarged pore on each side ( Fig. 18A, C, E, G View Fig. 18 cf. Fig. 16A View Fig. 16 , C-D); receptacular cluster larger, not divided into two lateral subclusters, its individual vesicles (especially posterior ones) longer ( Fig. 18B, D, F, H View Fig. 18 cf. Fig. 16B, E View Fig. 16 ).
Description of male (holotype): Colour in alcohol (much darker in life; see Fig. 2B View Fig. 2 for paratype): Carapace with wide grey-brown margin (broken posteromedially) including most of pars cephalica and connected to flower-shaped patch of same colour around fovea; three very light brown areas between eye mound and fovea (anterior two small, kidney-shaped and in a pair, posterior one larger, inverted lanceolate) and light patches between darker central and marginal areas. Chelicerae with proximal portion cream-coloured, distal portion grey-brown. Palps in proximal half of femur and tibia and in distal half of patella light brown, mottled with dark spots, other parts grey-brown; cymbium entirely reddish brown. Legs mostly grey-brown except for light brown distal half of all patellae and light brown proximal half of all femora (mottled with dark spots); leg tibiae (in contrast to those of palp) to tarsi entirely dark brown. Membranous parts of opisthosoma light brown; tergite I almost entirely grey-brown, tergite II with extensive dark lateral and median patches, following tergites with distinct dark lateral spots and increasingly indistinct median ones, last two tergites tiny and mostly grey-brown.
Bristles on carapace: Few short, weak bristles along most of its margins (completely absent posteriorly and largely absent laterally), on coxal elevations and in front of fovea (not behind); longer, stronger bristles on, behind and in front of eye mound.
Cheliceral teeth: Eleven small ones on promargin of cheliceral groove of each chelicera.
Scopula: Tarsi I-III with moderately dense scopula in distal half of ventral side, only distally divided by a short median stripe; tarsus IV with weak scopula in distal quarter, medially divided for its entire length.
Claws: Paired tarsal claws with 3-4 denticles on anterior legs, 2-4 denticles on posterior legs; unpaired claws with 1-2 indistinct denticles on tarsi I-III, none on tarsus IV. Palp: Tibial apophysis situated distally, only slightly set back from anterior margin of tibia ( Fig. 17A View Fig. 17 ), deeply divided:(a) longanddeepretrolateralpartpointingslightly away from axis of tibia, carrying three medium-long and bent megaspines; (b) short and small retrodorsal part carrying a single, slightly curved megaspine ( Fig. 17I View Fig. 17 ); long bristle retroventrally below tibial apophysis very strong, almost as thick as megaspines ( Fig. 17A, I View Fig. 17 ). Distal margin of cymbium with short lobes ( Fig. 17B View Fig. 17 , showing paratype matured 4.X.2012). Paracymbium globular, quite short and shallow, without retrolateral heel ( Fig. 17A, I View Fig. 17 ); cumulus indistinct, carrying a group of long stiff bristles ( Fig. 17A, I View Fig. 17 ). Subtegulum without apophysis. Tegulum with moderately wide, strongly dentate proximal edge ( Fig. 17C View Fig. 17 ). Contrategulum without recognizable ventral process; prolateral surface with a few ribs; distal edge sharp throughout, with an indistinct, very short and wide invagination (not a gap) prodorsally ( Fig. 17B View Fig. 17 , showing paratype matured 4.X.2012) before reaching very narrow, beak-shaped dorsal apex ( Fig. 17G View Fig. 17 ). Para-embolic plate large, its distal margin wide, strongly dentate ( Fig. 17A, C, G View Fig. 17 ); embolus proper slightly inclined prolaterad, relatively narrow ( Fig. 17A View Fig. 17 , C-D), dorsal and ventral walls of sclerotised part equally wide and lying close to each other, retrolateral wall with few indistinct longitudinal ridges ( Fig. 17G View Fig. 17 ); membranous part of embolus proper narrow, indistinct ( Fig. 17B View Fig. 17 , showing paratype matured 4.X.2012).
Measurements: Total length 11.57; carapace 5.08 long, 4.49 wide; opisthosoma 4.25 long, 2.91 wide; eye mound 0.80 long, 0.97 wide; palpal coxae 1.61 long, 1.10 wide; labium 0.43 long, 0.98 wide; sternum 2.24 long, 1.57 wide (0.87 on ventral surface); palp 9.10 long (2.72 + 1.65 + 3.31 + 1.42); leg I 15.36 long (4.13 + 1.97 + 3.31 + 3.98 + 1.97); leg II 15.83 long (4.17 + 2.01 + 3.31 + 4.33 + 2.01); leg III 17.32 long (4.41+ 2.05 + 3.46 + 5.04 + 2.36); leg IV 22.91 long (5.67 + 2.20 + 4.88 + 7.01 + 3.15).
Description of female (allotype): Colour in alcohol (much darker in life): Generally darker reddish-brown than male; light areas on carapace mottled with dark brown spots, flower-shaped area around fovea less clearly outlined; tarsi and metatarsi of legs and palps mostly dark, with a small light zone at base of palpal tarsi and leg metatarsi; all tibiae with dark proximal and subdistal annulations; femora with indistinct (in comparison to tibiae) and broken proximal and subdistal annulations; opisthosomal tergites II-V with larger dark median patches; genital area darker than surrounding parts of genital sternite, with a light posterior margin. Bristles on carapace: As in male, plus several tiny bristles behind fovea.
Cheliceral teeth: Eleven mostly strong cheliceral teeth on promargin of left cheliceral groove, nine on right.
Claws: Palpal claws with two denticles. Paired claws with 2-3 denticles on anterior legs, 2-4 denticles on posterior legs; unpaired claws with two denticles on tarsi I-III, none on tarsus IV. All tarsi without scopula.
Vulva: Posterior margin of genital sternite slightly and widely invaginated. Vulval plate ( Fig. 18 View Fig. 18 , illustrations of paratypes) with pigmentation much reduced but still discernible in median zone. Posterior stalk reduced to a very weakly pigmented, wide to narrow anterior part and a small, strongly pigmented, ventrad-bent posterior sclerite at some distance from posterior margin of genital sternite. Genital atrium with numerous hairs on both sides of remnant of posterior stalk. Lateral and posterior margins of poreplate indistinctly outlined, not bulging from ventral side; anterior margin only slightly invaginated at midpoint, not forming anterolateral lobes. CDO relatively small, longitudinally slit-like or semicircular, lying in weakly pigmented area of poreplate ( Fig. 18A, C, E, G View Fig. 18 , showing paratypes). Ventral receptacular cluster quite large and racemose, not divided into two lateral subclusters, its individual vesicles mostly digitiform, longer than wide ( Fig. 18B, D, F, H View Fig. 18 , showing paratypes).
Measurements: Total length 19.45; carapace 6.61 long, 5.67 wide; opisthosoma 8.27 long, 6.69 wide; eye mound 0.95 long, 1.01 wide; palpal coxae 2.20 long, 1.54 wide; labium 0.75 long, 1.73 wide; sternum 3.15 long, 2.20 wide (1.26 on ventral surface); palp 11.61 long (3.78 + 2.20 + 2.83 + 2.80); leg I 14.25 long (4.49 + 2.48 + 2.95 + 2.87 + 1.46); leg II 14.68 long (4.49 + 2.56 + 2.95 + 3.11 + 1.57); leg III 15.43 long (4.57 + 2.56 + 2.99 + 3.58 + 1.73); leg IV 22.44 long (6.30 + 2.91 + 4.57 + 6.14 + 2.52).
Variation: Carapace lengths in males (n=10) 4.50- 6.83, carapace widths 3.98-6.21; in females with welldeveloped vulval plates (n=18) 5.55-7.24 and 4.80- 6.30, respectively. All ten males possess a weak ventral scopula on tarsus IV; it is medially divided in three of them (including the holotype), undivided in the others. Three males have more or less distinct remnants of “tibial spurs” (sensu Platnick & Goloboff, 1985) on legs I-III. A male lacks one of its AME; in all other specimens examined both AME are well-developed. Variation in details of the male palp, see Fig. 17 View Fig. 17 G-H, I-K; variation in the morphology of the vulval plate, see Fig. 18 View Fig. 18 . One of the larger females examined has a small knob-like sclerite in the membranous dorsal wall of its vulva which fits into the CDO of the ventral wall (= vulval plate). Such a structure was also observed in some (but only few) vulvae of large females of other species. This is an unlikely (because inefficient) plugging device and more probably a by-product of increased sclerotisation of the vulval plate in old females.
Distribution: Known only from the type locality ( Fig. 1 View Fig. 1 , locality 14) in the deep south of Thailand. This area lies in the former Sultanate of Pattani which was an independent Malay kingdom until 1785, then became a tributary of the kingdom of Siam, and in 1909 formally a Siamese province. A second species, Liphistius cf. thaleban Schwendinger, 1990 , occurs at the same locality. That species is much larger, has orangecoloured femora in larger juveniles and adult females, and belongs to the trang -group.
Biology: The spiders examined were collected in an evergreen rain forest, close to a stream. Their burrows had a single trapdoor, usually opening downwards, and 8-10 (mostly nine) relatively short signal lines (up to 4 cm long) spread over soil surface. Trapdoors of penultimate males (n=8) were 1.25-1.8 cm long and 1.65-2.3 cm wide, those of females (n=17) up to 2.1 cm long and 3.0 cm wide.
Males matured in captivity between August and November, most (six out of ten) in September. Adult females in captivity usually moulted twice per year, in January to April and in June to November. As all females were collected in July and October (outside the breeding season), no egg cases were observed.
Upon being provoked, one mature male and one large female displayed “tiptoing” but without the pumping movements (raising and lowering the body above the surface) usually observed in large Liphistius spiders.
Two females carried mites of the genus Ljunghia (see Halliday & Juvara-Bals, 2016: 857) which left clearly visible dark bite marks on the carapace and on the light proximal portion of the chelicerae, but not on other parts of the body. The mites were seen to aggregate in the fovea, around the sternum, under the spinnerets and on the ventral side of the leg femora of the spiders. One mite was seen inserting its gnathosoma into one of the bite marks on the carapace, obviously taking in food.
Two immature males (with swollen palpal tarsi and thus possibly penultimate; not paratypes) had a swollen, lightcoloured (like porcelain) opisthosoma when collected and died soon afterwards. This indicates an infection with Rickettsiales (see Haupt, 2003: 66-67, fig. 41D). Haupt (2003: 67, figs 24A, 25A) reported rickettsia-like microorganisms in the spermophore of the mesothelid Ryuthela nishihirai ( Haupt, 1979) and assumed that infection can occur through copulation. This was certainly not the case in the two immature males of L. indra sp. nov.
At the type locality, L. indra sp. nov. occurs together with Liphistius cf. thaleban . Burrows of both species were found side by side.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |