Leptophis nigromarginatus ( Günther, 1866 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5153.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A658ADE4-F352-4D16-9DC7-2721BCBE1EEF |
|
persistent identifier |
https://treatment.plazi.org/id/039B220B-FFD5-D17C-FF6B-95C4FD38EA31 |
|
treatment provided by |
Plazi |
|
scientific name |
Leptophis nigromarginatus ( Günther, 1866 ) |
| status |
|
Leptophis nigromarginatus ( Günther, 1866) View in CoL
( Figs. 11G–J View FIGURE 11 , 23–24 View FIGURE 23 View FIGURE 24 , 26 View FIGURE 26 )
Ahaetulla View in CoL nigromarginata Günther, 1866: 28. Female holotype ( BMNH 1946.1.5.7; examined). Type locality: “ Upper Amazons ” .
Leptophis ortoni Cope, 1876: 177 . Syntypes (ANSP 25774 and ANSP 25775; not examined). Type locality: “Solmoens [= Solimões River] or middle Amazon”; Amaral 1930a: 16. New synonym.
Leptophis ahaetulla View in CoL — Griffin 1916: 184 (in part) (Griffin listed four specimens deposited in Carnegie Museum as L. ahaetulla , but the CM 366 is here assigned to L. nigromarginatus ); Wallach et al. 2014: 372 (in part).
Leptophis nigromarginatus View in CoL — Griffin 1916: 185; Amaral 1925: 18-19; Torres-Carvajal & Terán 2021: 2.
Leptophis occidentalis nigromarginatus — Amaral 1930a: 16; Amaral 1930b: 85; Amaral 1930c: 162.
Leptophis ahaetulla nigromarginatus — Oliver 1942: 4; Daniel 1949: 310; Amaral 1977: 78; Peters 1960: 526; Peters & Orejas- Miranda 1970: 163; Pérez-Santos & Moreno 1988: 207; Pérez-Santos & Moreno 1991: 216; Tipton 2005: 161
Leptophis ahaetulla nigromarginata (sic); Dixon & Soini 1977: 58; 1986: 113; Jorge da Silva Jr. 1993: 61.
Leptophis ahuetulla (sic) nigromarginatus — Duellman 1978: 249.
Leptophis ahaetulla ortoni — Oliver 1942: 4; Peters & Orejas-Miranda 1970: 163; Pérez-Santos & Moreno 1988: 212.
Leptophis ahaetulla View in CoL [ nigromarginatus ]— International Commission of Zoological Nomenclature 1958: 270.
Diagnosis. Leptophis nigromarginatus can be distinguished from its congeners by the following unique combination of character states: (1) head scales edged with black (more prominent in specimens from the western portion of its range) and occasionally one large black spot present in center of each parietal and supraocular scales; (2) adult color pattern with no dark dorsal bands; (3) dorsum greenish blue or dark blue and dorsal scales with prominent black margins; (4) keeled dorsal scales, except first dorsal row on each side; keels of dorsal scales not black; (5) no loreal scale; (6) ventrals 144–172 in males, 146–182 in females; (7) subcaudals 129–179 in males, 146–182 in females; (8) dorsal scales of tail with no keels; (9) maxillary teeth 23–26; (10) TL/ SVL: 95% CI = 0.640 –0.649 (n = 119); (11) small spines at first basal row of hemipenial body; (12) calyces more developed on sulcate side of hemipenial body GoogleMaps .
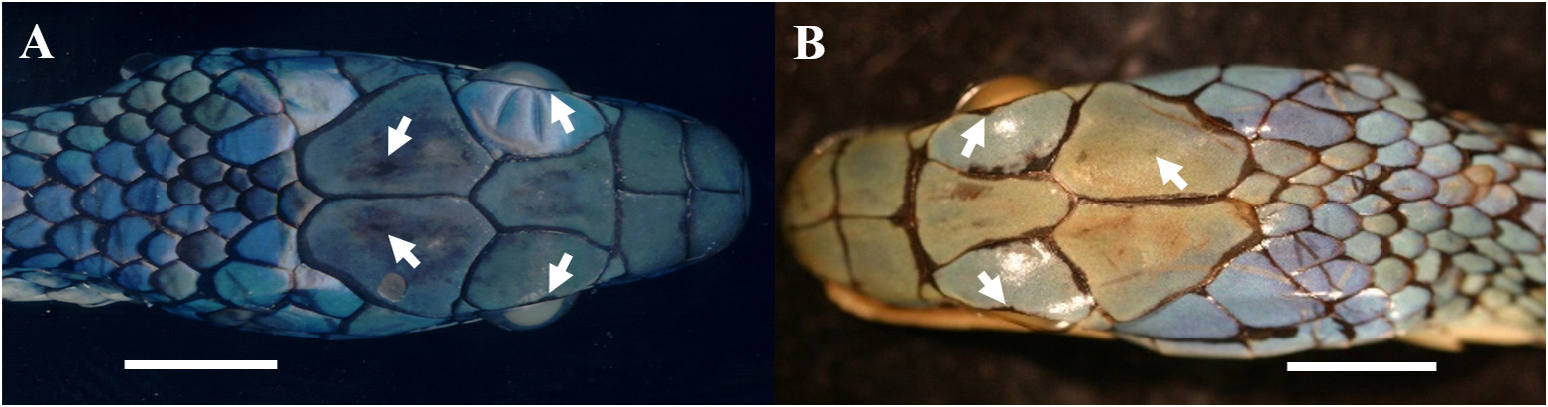
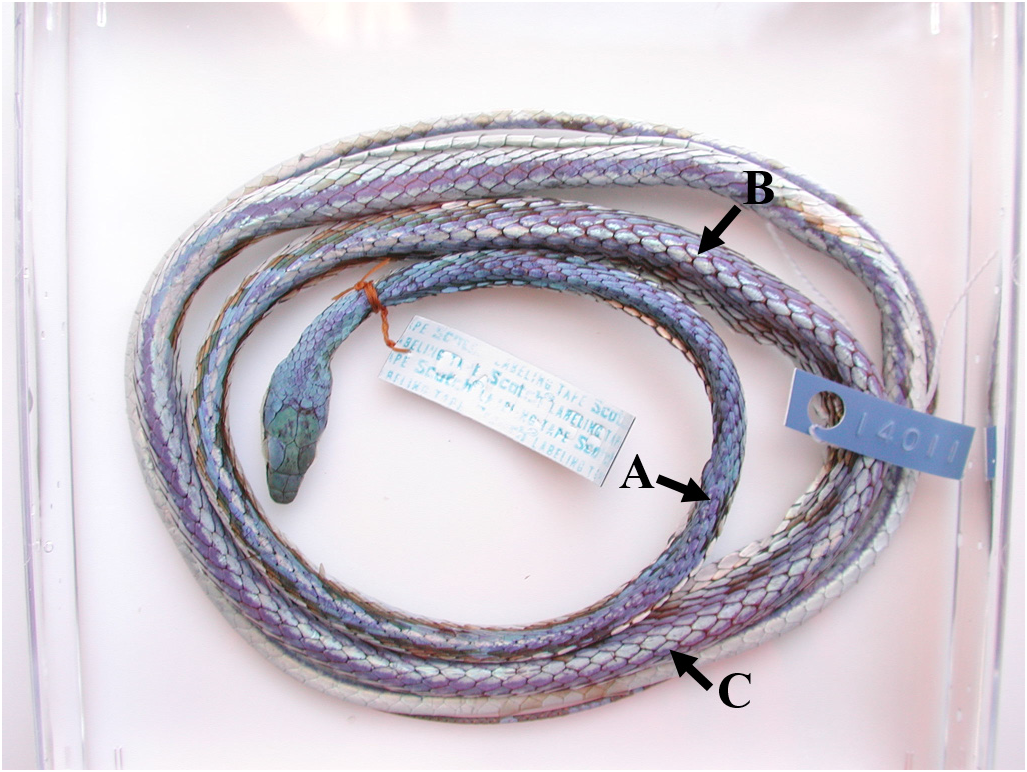
Comparisons. Leptophis nigromarginatus differs from all members of the L. ahaetulla complex by the combination of head scales edged with black—more prominent in specimens from the western portion of its range—and occasionally one or two black spots in center and outer edge of each parietal and supraocular scale, respectively ( Figs. 23A View FIGURE 23 , 24A–C View FIGURE 24 , 26 View FIGURE 26 ) (vs. head scales not edged with black and with no black spot in parietals and supraoculars). Leptophis nigromarginatus differs from the sympatric L. ahaetulla by having ventrals edged anteriorly and laterally with Robin’s Egg Blue (161) ( Figs. 24B, D View FIGURE 24 ) or dark greenish blue in preservative ( Fig. 23B View FIGURE 23 ) (vs. ventrals immaculate) and by absence of vertebral stripe ( Fig. 23C–D View FIGURE 23 ) (vs. vertebral stripe present, with coloration spreading out laterally, and occupying scales of paravertebral rows in the posterior portion of the body ( Figs. 1 View FIGURE , 3 View FIGURE ). Adults of L. nigromarginatus have higher values of TL/SVL—95% CI = 0.640 –0.649 —than L. occidentalis (vs. 95% CI = 0.600 –0.626) ( Table 2).
Variation and sexual dimorphism. Largest male SVL 835 mm, TL 526+ mm and largest female SVL 822 mm, TL 456 mm; ventrals 144–172 in males (152.3 ± 4.9, n = 158), 146–182 in females (156.5 ± 4.4, n = 207). Subcaudals 129–179 in males (150.1 ± 8.3, n = 96), 133–166 in females (148 ± 7.8, n = 125); supralabials 6–10 (8.5 ± 0.5, n = 672), with fifth–sixth (55.2%, n = 372), fourth–fifth (44.3%, n = 298), or, rarely, fifth–sixth–seventh (0.3%, n = 2), sixth–seventh (0.1%, n = 1), and third–fourth (0.1%, n = 1) bordering orbit; infralabials 7–12 (10.4 ± 0.7, n = 672), with first 6 (59.8%, n = 402), first 5 (38.4%, n = 258), or, rarely, first 7 (0.9%, n = 6), and first 4 (0.6%, n = 4), and first 8 (0.3%, n = 2) contacting first chin shields; preoculars 1–2 (1.0 ± 0.2, n = 674); postoculars 1–3 (2.0 ± 0.2, n = 674); anterior temporal 1 (n = 331) and six specimens with 2 on both sides; posterior temporal 1–2 (1.8 ± 0.4, n = 672), and a single specimen with 3 on left side; keels more developed in adult males than females and juveniles; keels present on dorsal scale rows II to XIV in males, and VI to X in females; parietal and supraocular scales with black spots less prominent in specimens from eastern portion of its range (e.g., MCZ 2792 About MCZ ) .
The specimen AMNH 56141 presents keels on dorsal scales of the tail until the point of reduction from six to four rows. The specimen USNM 238382 has keels on dorsal scales of the tail (about 135 mm of TL). Nine specimens: AMNH 52472, a male with 462 mm in total length, AMNH 54116, a male with 314 mm in total length, AMNH 56026, a female with 288 mm in total length, AMNH 56035, a female with 280 mm in total length, LSUMZ 26805, a male with 322 mm in total length, LSUMZ 26801, a female with 302 mm in total length, FMNH 218506, a male with 318 mm in total length, and LSUMZ 26805, a male with 322 mm in total length with bands on anterior and middle portions of the body, and LSUMZ 26804, a female with 620 mm in total length with bands only on anterior portion of the body. Females have more ventrals (F 1,365 = 72.3792; P <0.01) and subcaudals (F 1, 221 = 3.8270; P=0.0487) than males. The TL/SVL showed no significant difference between females and males (F 1,217 = 1.0653; P = 0.3037).
Hemipenial morphology. Four retracted organs examined extend to the level of sixth subcaudals. Everted hemipenis unilobed, noncapitate; sulcus spermaticus centrolineal, undivided, extending from base to tip of lobe; basal portion bears small-sized spines, distributed approximately in 7 rows encircling the organ; first row bears 5–7 moderate-sized spines; most spines in the first row larger than those of other rows; one-two spines in the first row adjacent to sulcus spermaticus larger than adjacent spines; few minute spinules widely scattered on basal portion, occurring below first row of spines (LPHA 1731), or on basal and lateral portion of hemipenial body (LSUMZ 26800); minute spinules adjacent to sulcus spermaticus (TCWC 42177) as a extension of spinules present on basal portion; calyces ornamented with 5–6 papillae concentrated along distal portion of hemipenial body; calyces more developed on sulcate side (LSUMZ 26800); papillae gradually decrease in number and size toward distal portion of hemipenis; distal portion of lobe may be either completely calyculate (FMNH 231776), or with tiny nude area, situated at the end of sulcus spermaticus (LPHA 1731) ( Figs. 11G–J View FIGURE 11 ).
Coloration in life. Supracephalic scales Yellowish Spectrum Green (128) and edged with black (more prominent in specimens of the western portion of the range); usually one or two black spots (with one larger) in the center of each parietal scale; outer edge of supraoculars usually ornamented with one or two black spots; narrow postocular black stripe always reduced to black margin on posterior edge of lower postocular, lower edge of anterior temporal and lower posterior temporal, and upper edges of last three supralabials; dorsum and tail Yellowish Spectrum Green (128); dorsal coloration of the body occupies proportionately the same width throughout entire body length; all dorsal scales of the body and tail with prominent black margins; rows I–III (occasionally the I–V) on the anterior third of the body yellowish to Light Greenish Yellow (87), or Sulphur Yellow (80) in juveniles; supralabials white to Light Turquoise Green (146); infralabials, chin and throat white; ventral scales usually edged anteriorly and laterally with Robin’s Egg Blue (161). Duellman (2005, figs. 205 and 206) illustrated and described the color pattern of L. nigromarginatus (as L. ahaetulla ). Duellman (1978) noted that juveniles and some adults of Ecuador have extensive coppery coloration on the body, similar to L. cupreus . Dixon & Soini (1986: 113) and Duellman (1978: 249) briefly described the color pattern of L. a. nigromarginatus . Pérez-Santos & Moreno (1991, fig. 90) and Campbell & Lamar (2004, fig. 1175) illustrated specimens of L. a. nigromarginatus from Peru and Ecuador, respectively.
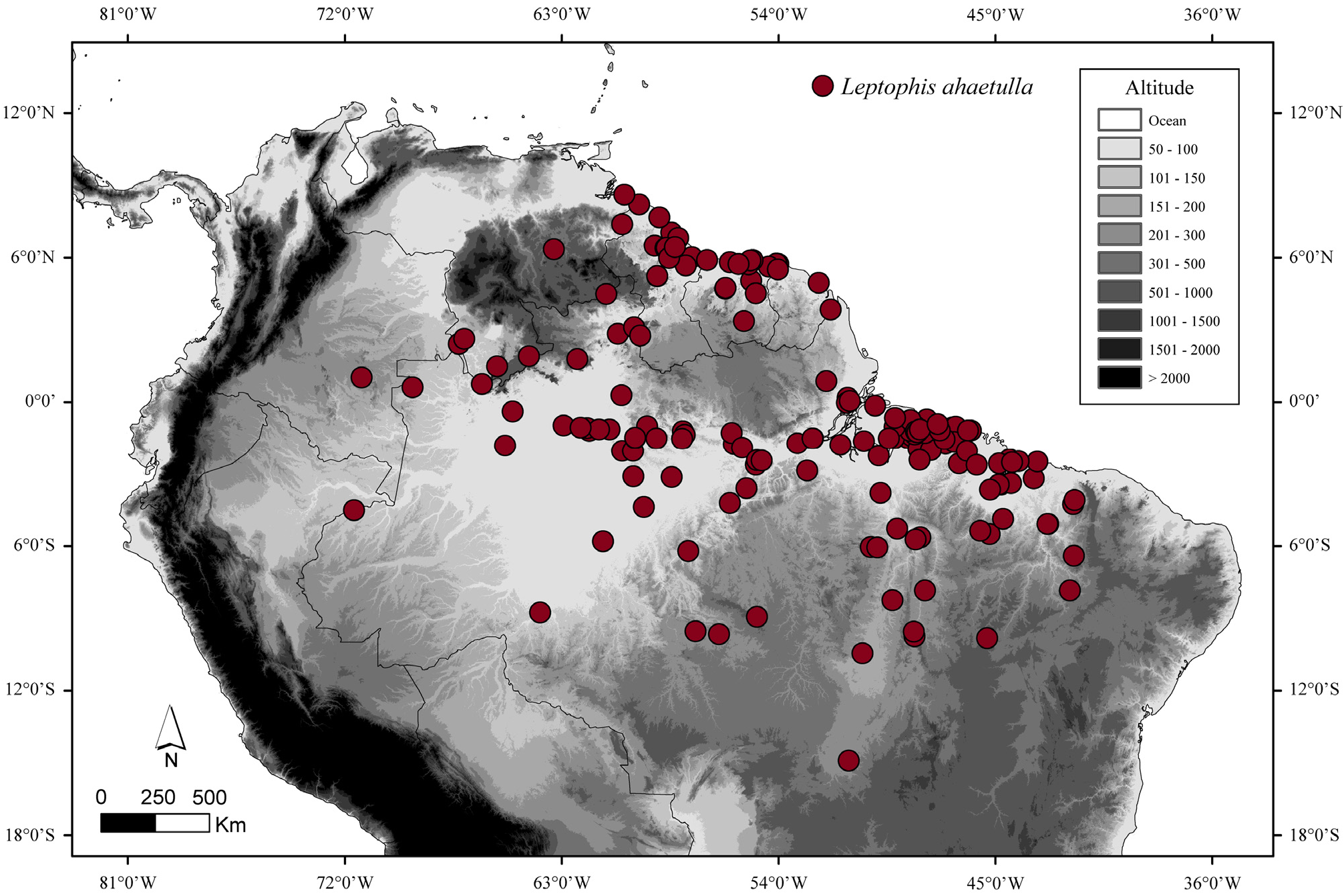
Distribution and natural history. Leptophis nigromarginatus is known from Guyana, the western and middle Amazon regions of Brazil, the Amazonian lowlands of Colombia, Ecuador, and Peru, and extreme northern Bolivia. These snakes were collected up to 700 m asl in the tropical and subtropical moist broadleaf forests and tropical and subtropical dry broadleaf forests ecoregions, as defined by Olson et al. (2001) ( Fig. 18 View FIGURE 18 ). William W. Lamar (pers. comm., 26 Aug 2015) observed one specimen of L. nigromarginatus in the Iquitos region in the act of consuming an anuran of the genus Osteocephalus Fitzinger.
Remarks. The supracephalic scales edged with black and dorsal coloration of a male (AMNH 60792) collected in Karananbo, Guyana are similar to that of L. nigromarginatus . The occurrence of L. nigromarginatus in Guyana is completely outside the previously known range of this species, extending the geographic distribution of this species about 830 km northwestward from the Brazilian municipality of Santarém, Pará, where this species also occurs (see below). Leptophis ahaetulla and L. nigromarginatus are distributed simpatrically through the States of Pará and Amazonas, in northern Brazil ( Figs. 4 View FIGURE 4 , 18 View FIGURE 18 ). Specific information on the type locality of the latter species was not found. Actually, it is difficult to infer the actual provenance of the type specimen since the collector, Edward Bartlett, arrived at the Brazilian state of Pará in 1895 and made journeys up to Ucayali and Huallaga Rivers and to nearby areas ( Palmer 1944).
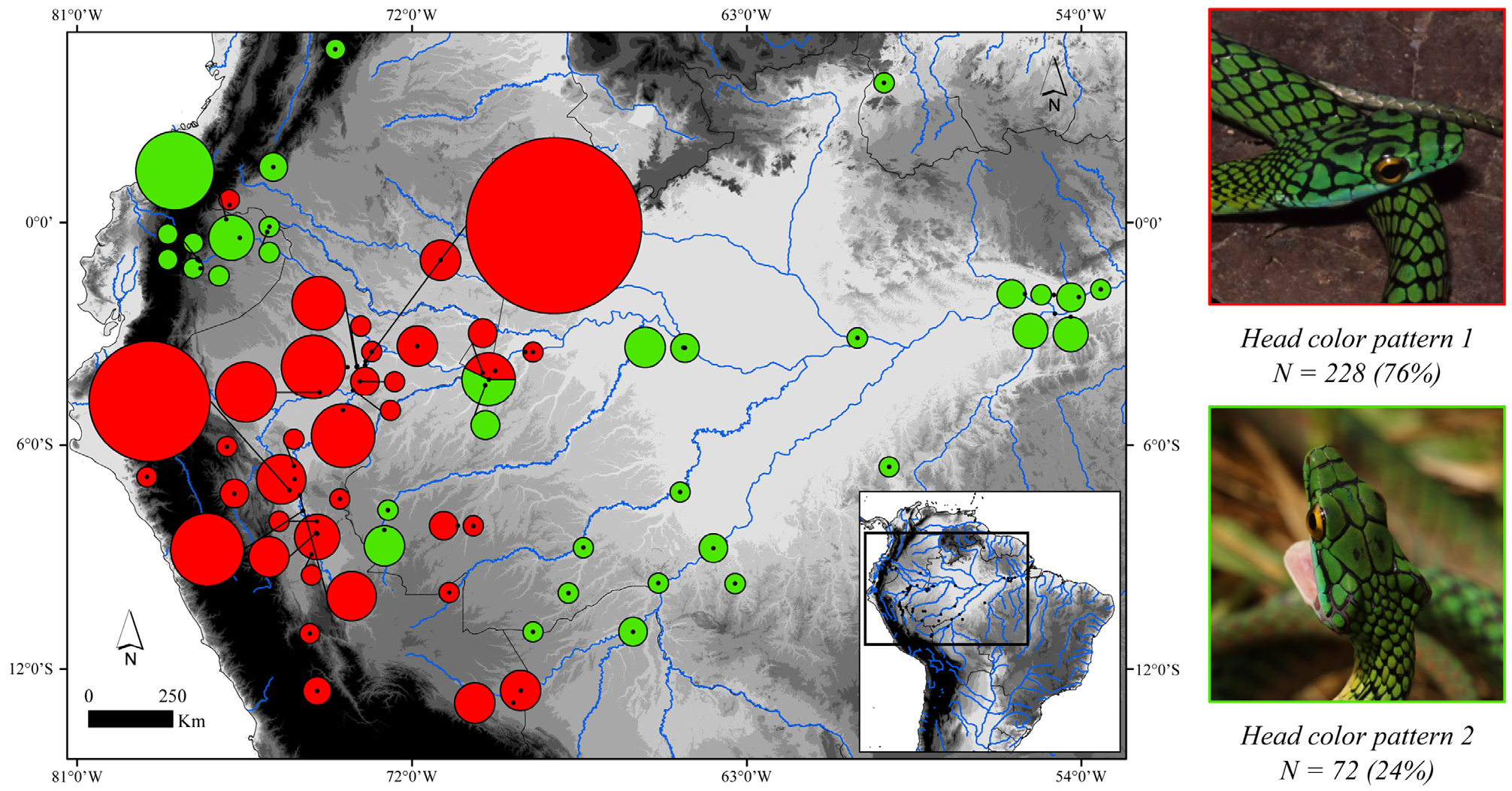
Gradual decrease in dark pigment on ventral scales in populations of Leptophis nigromarginatus from Peru and western Amazonia compared to those from western Pará state, Brazil, was used by Oliver (1948) as one of the characteristics to distinguish L. a. nigromarginatus from L. a. ortoni . Based on features of the head and dorsum color pattern, Oliver (1948: 239) recognized four color categories for L. nigromarginatus , all represented in different frequencies in the Peruvian populations. The fourth category (present in 7.5% of Peruvian populations) would include specimens with the same color pattern as noticed in L. a. ortoni from the western portion of the distribution range of the latter taxon, “with black borders on the head shields and many of the dorsal scales, without black spots on the head shields, with light ventral and lower dorsal coloration” (but see below). According to Oliver (1948: 199), there is a gradual decrease in the amount and distribution of black pigment on the borders of the head scales and on the dorsal scales of the trunk from the populations of L. nigromarginatus (a typical Peruvian subspecies in Oliver’s sense) to populations of L. a. ortoni from the middle Amazonia, whose head scales would frequently have no black borders. Together with L. a. ahaetulla , these populations would be connected along the Amazon basin by a series of populations that gradually change in color from one extreme to the other along a west-east gradient. These supposed clines were related to a progressive change in temperature and humidity, although the causal relationship was unclear ( Oliver 1948: 199). However, as Mayr (1992) pointed out, the more distant two populations (of the same species) are from each other, the more likely they are to differ in a number of characteristics. Dorsal scales rows I-III of live specimens illustrated in Fig. 24A–B View FIGURE 24 are yellowish to Light Greenish Yellow (87) (Oliver´s light ventral and lower dorsal coloration), or Sulphur Yellow (80) in the juvenile specimen, suggesting a distinct ontogenetic color change in L. nigromoarginatus , as also observed in L. bocourti and L. occidentalis . Some specimens captured along the typical distribution of L. a. ortoni (BMNH 97.12.29.15, Manaus and UMMZ 56304, Óbidos) exhibit the color pattern of a predominantly uniform greenish blue or dark blue occupying scales of all dorsal rows, except I–III, as in L. nigromarginatus . Oliver (1948: 239, 246) stated that the ventrals of L. nigromarginatus and L. ortoni are patterned “with irregular dark outer and anterior margin virtually same color as on dorsal scales [i.e., greenish blue or dark blue]” and “with prominent bluish green or dark blue anterior and outer margins”, respectively; both patterns here assumed as indistinguishable from each other. The ventral scales of the holotype of L. nigromarginatus ( Fig. 23B View FIGURE 23 ) are still edged with greenish blue as well as the ventrals of BMNH 97.12.29.15 and UMMZ 56304. It is worth noting that the holotype of L. nigromarginatus comes from an unspecified locality. Although female KU 112279 (color image KUDA 013700) and male KU 155512 (color image KUDA 013701) ( Fig. 23C–D View FIGURE 23 , respectively) were collected in the Ecuadorian locality of Santa Cecília, both show distinct differences in the prominence of supracephalic black borders and spots, being both more prominent in the female specimen. On the other hand, the specimen from Croa River, municipality of Cruzeiro do Sul, state of Acre ( Fig. 24C–D View FIGURE 24 ), shows spots on its parietal and supraocular scales not as prominent as in L. nigromarginatus . We detected basically two-color patterns ( Fig. 25 View FIGURE 25 ) related to the prominence of black borders and spots on the supraocular and parietals, with a certain degree of overlap in both. The first pattern, herein named “head color pattern 1”, is present in 228 (76%) specimens examined from Peru, southern Colombia, and the western portion of the Brazilian Amazonia; specimens within this pattern are characterized by having black edges on supracephalic scales more heavily edged, one or two black spots in center of each parietal, and one black spot on outer edge of supraocular scales ( Fig. 25 View FIGURE 25 , red circles). The second pattern, herein named “head color pattern 2”, is present in 72 (24%) of the specimens from southern Colombia, western and eastern portions of the Brazilian Amazonia, and is similar to the first pattern, although with the edges of supracephalic scales less prominent and the black spots smaller. Moreover, several specimens with “head color pattern 2” occur at the northwestern limit (i.e, Ecuador and Colombia) of the range of specimens showing “head color pattern 1” ( Fig. 25 View FIGURE 25 , green circles). On the other hand, the supracephalic scales of LPHA 1731 from Santarém, state of Pará, Brazil are slightly edged with black, while parietals and supraoculars are ornamented with one black spot each ( Fig. 26A View FIGURE 26 ), and all dorsal scales are edged with black. A similar condition can be noticed in MCZ 2792 ( Fig. 26B View FIGURE 26 ), a specimen also collected in the same locality, in which Oliver’s fourth (or even third) category would occur. Our results suggest that, as in other snake species (e.g., Moraes et al. 2016; Gibbs et al. 2018), the Amazon River and its tributaries are not absolute barriers to gene flow between samples with “head color pattern 2”.
The size of basal spines might also distinguish L. nigromarginatus from L. a. ortoni , since the hemipenis of L. nigromarginatus is supposedly ornamented with five to six moderate-sized spines in the first basal row (vs. no large basal spines in L. a. ortoni ) ( Oliver 1948: 238, 245). However, the hemipenis illustrated in Fig. 11G–H View FIGURE 11 , belongs to a specimen (LPHA 1731) that was captured in Santarém, Pará State, Brazil, where Oliver (1948: 222) identified intergrades between L. a. ahaetulla x L. a. ortoni . The first row of basal spines of this organ is ornamented with small-sized spines (although small, they are slightly larger than the hemipenial spines of sympatric L. ahaetulla ), as in Oliver’s description of L. a. ortoni . Nevertheless, the basal spines of the first row of TCWC 42177 from Centro Union, Peru ( Fig. 11I–J View FIGURE 11 ), are similar in size to the spines of LPHA 1731, which makes the distinction between these subspecies difficult based on hemipenial characters.
The available evidence is therefore insufficient to objectively distinguish L. nigromarginatus from L. a. ortoni , and consequently we here consider L. a. ortoni a junior synonymy of L. nigromarginatus , extending the distribution of the latter to western portion of Pará State, Brazil.
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Leptophis nigromarginatus ( Günther, 1866 )
| Albuquerque, Nelson Rufino De & Fernandes, Daniel S. 2022 |
Leptophis ahuetulla
| Duellman, W. E. 1978: 249 |
Leptophis ahaetulla nigromarginata
| Dixon, J. R. & Soini, P. 1986: 113 |
| Dixon, J. R. & Soini, P. 1977: 58 |
Leptophis ahaetulla
| International Commission of Zoological Nomenclature 1958: 270 |
Leptophis ahaetulla nigromarginatus
| Tipton, B. L. 2005: 161 |
| Perez-Santos, C. & Moreno, A. G. 1991: 216 |
| Perez-Santos, C. & Moreno, A. G. 1988: 207 |
| Amaral, A. 1977: 78 |
| Peters, J. A. 1960: 526 |
| Daniel, H. 1949: 310 |
| Oliver, J. A. 1942: 4 |
Leptophis ahaetulla ortoni
| Perez-Santos, C. & Moreno, A. G. 1988: 212 |
| Peters, J. A. & Orejas-Miranda, B. 1970: 163 |
| Oliver, J. A. 1942: 4 |
Leptophis occidentalis nigromarginatus
| Amaral, A. 1930: 16 |
| Amaral, A. 1930: 85 |
| Amaral, A. 1930: 162 |
Leptophis ahaetulla
| Wallach, V. & Williams, K. L. & Boundy, J. 2014: 372 |
| Griffin, L. E. 1916: 184 |
Leptophis nigromarginatus
| Torres-Carvajal, O. & Teran, C. 2021: 2 |
| Amaral, A. 1925: 18 |
| Griffin, L. E. 1916: 185 |
Leptophis ortoni
| Amaral, A. 1930: 16 |
| Cope, E. D. 1876: 177 |
Ahaetulla
| Gunther, A. 1866: 28 |