Labeobarbus, : CURRENT
|
publication ID |
https://doi.org/ 10.1111/zoj.12366 |
|
persistent identifier |
https://treatment.plazi.org/id/03A187AA-FFFB-950C-FF5C-FE10FF47FCBC |
|
treatment provided by |
Marcus |
|
scientific name |
Labeobarbus |
| status |
|
LABEOBARBUS: CURRENT View in CoL View at ENA STATE OF THE ART
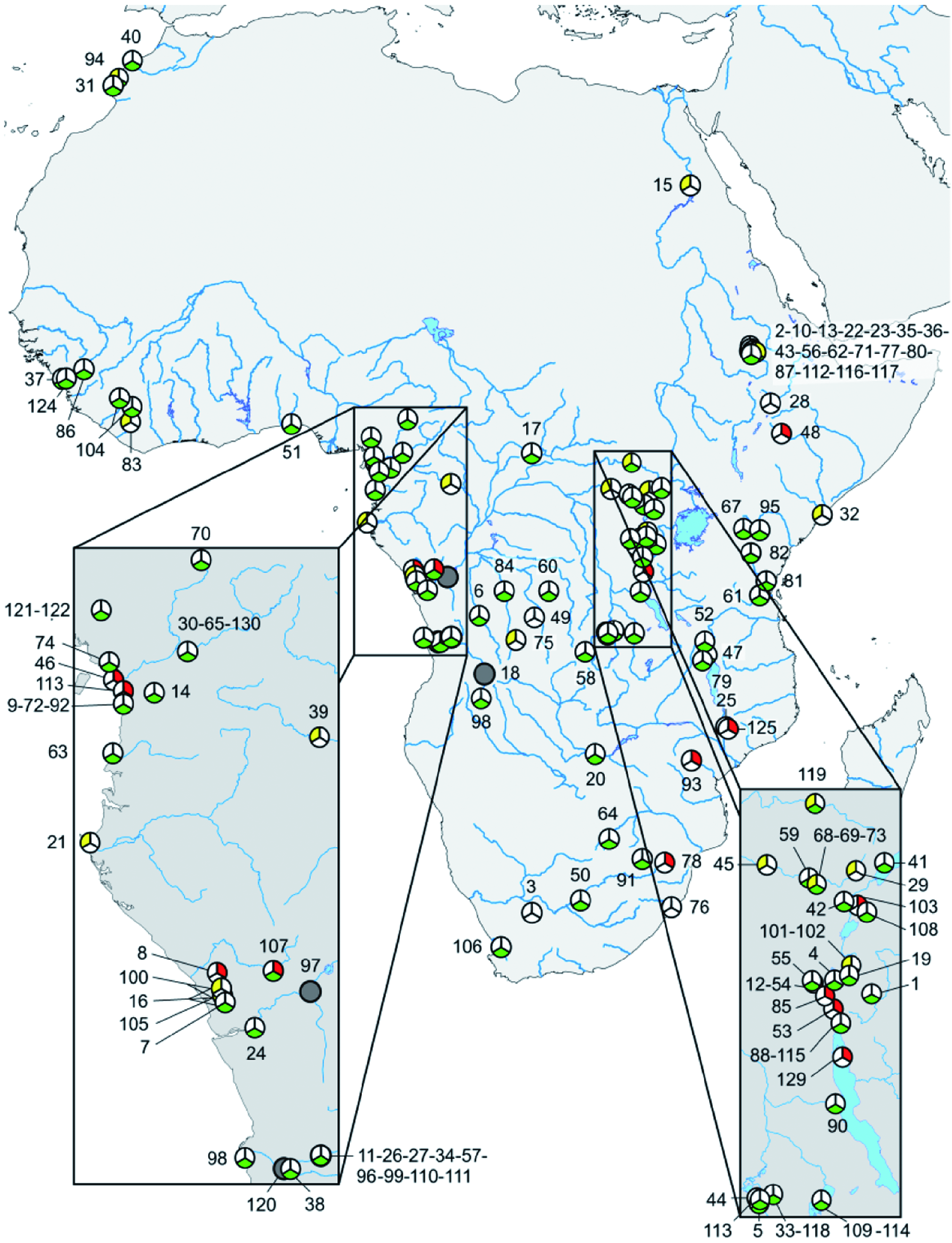
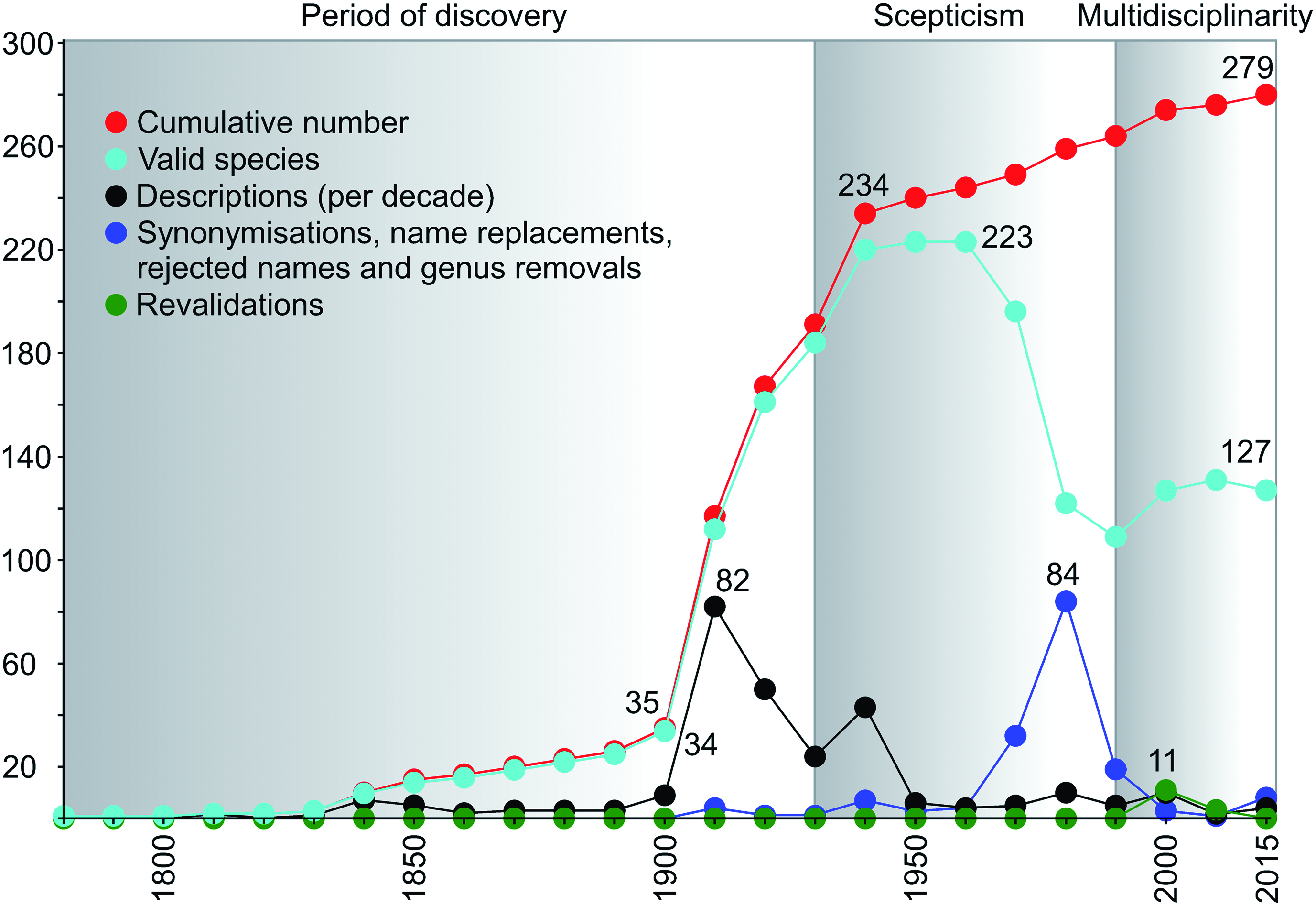
In light of all the aforementioned new developments in the field (see Tsigenopoulos et al., 2010; Borkenhagen & Krupp, 2013; Levin et al., 2013; Berrebi et al., 2014; Borkenhagen, 2014), the need for a comprehensive list of all species-level taxa to be included in or excluded from Labeobarbus s.l. has become an important prerequisite for any further studies of the African large hexaploid Torini, in particular, and the African and Middle East ichthyofauna in general. Therefore, a table listing all currently valid African Labeobarbus s.l., Acapoeta , and Sanagia species has been compiled (see Table 1). For details with regards to how data have been compiled, see the Material and methods section and the legend to Table 1.
The compilation of the present overview: what, how, and why?
Our current compilation lists a total of 125(+2) valid African Labeobarbus s.l. species known to date ( Table 1). Mouth phenotype diversity has been tabulated with the two major hypotheses in mind: on one hand, the two morphologically well-documented cases of ‘intergeneric’ hybridization cited above, where Banister (1972, 1976a) illustrated intermediate mouth phenotype(s) to represent hybrid specimens; and on the other hand, the decision by Banister (1972) and others ( Groenewald, 1958; Jubb, 1963, 1967, 1968; Crass, 1964) to interpret the huge observed mouth phenotype variation, encompassing both the Labeobarbus as well as the Varicorhinus mouth phenotypes, as intraspecific variation. The latter way of looking at the observed mouth phenotype diversity has also resulted in this aspect becoming somehow largely invisible, as it is presumed to be largely uninformative or even irrelevant for species diagnosis. Therefore, the current tabulation should be seen as a first effort in mapping the terrain, and has been undertaken to give a first glimpse of the current problems, questions raised, and further research needed.
The present overview: details on the taxonomic decisions made
To fully understand the extent and taxonomic conclusions of the provided list, the following points are explained in greater detail: (1) species previously explicitely allocated to Labeobarbus but which have been removed; (2) the identification of implicit synonymies according to the literature; (3) a small Barbus revealed to be a Labeobarbus species ; (4) Labeobarbus spp. identified as possible hybrid phenotypes in the literature; (5) the neotype designation for L. beso , previously V. beso , the type species of the genus Varicorhinus ; (6) substitute names ( ICZN, 1999: article 60) following the synonymization of Varicorhinus with Labeobarbus ; (7) the lectotype designation for Labeobarbus sandersi ( Boulenger, 1912) , previously a Varicorhinus species ; (8) generic level synonyms of Labeobarbus for Africa.
1. Six Ethiopian species reported by Getahun (2007a: 94) as belonging to the genus Labeobarbus were, in fact, attributed to it in error and should have remained in Barbus (now Enteromius ; see Yang et al., 2015). These are: Barbus anema Boulenger, 1903 ; Barbus arambourgi Pellegrin, 1935 ; Barbus kerstenii Peters, 1868 ; Barbus neglectus Boulenger, 1903 ; Barbus stigmatopygus Boulenger, 1903 ; and Barbus werneri Boulenger, 1905 (actually a junior synonym of B. stigmatopygus : see annotated checklist 1). Indeed, all these are small Barbus , now Enteromius , with radiately striated scales and a dorsal fin formula of III7–8. One of them, i.e. B. kerstenii , even has a spiny, serrated, last unbranched dorsal fin ray, a character state never found in Labeobarbus . Also, the attribution of Barbus litamba Keilhack, 1908 to Labeobarbus by Snoeks (2004) is in error. Although this is indeed a large Barbus (maximum size, 315 mm SL; see Lévêque & Daget, 1984), this species also has a spiny, serrated, last unbranched dorsal fin ray, which, as stated above, is a character state that has never been documented for any of the karyotyped, hexaploid, Labeobarbus . Instead, the few karyotyped large African Barbus with a spiny, serrated, last unbranched dorsal fin ray were all shown to be diploid, as for Barbus mattozi Pereira Guimarães, 1884 , or tetraploid, as for B. capensis (under Barbus andrewi Barnard, 1937 ) and Barbus serra Peters, 1864 (see Tsigenopoulos et al., 2002). The same holds true for Barbus rapax Steindachner, 1894 (actually a junior synonym of B. mattozi following Jubb, 1963, although questioned by Skelton, 2001: 161), a sawfin Barbus (see Skelton, 2001) with radiating striae that has also erroneously been identified as a yellowfish (now Labeobarbus ) by Groenewald (1958) in the past. Furthermore, following E. Vreven, E.R. Swartz & P.H. Skelton (unpubl. data), B. capensis has to be removed from Labeobarbus as a re-examination of the holotype revealed it to be a senior synonym of B. andrewi and hence not a Labeobarbus species. Therefore, in the present list, the name of L. seeberi , previously a junior synonym of B. capensis , has been used for the southern African clanwilliam yellowfish.
Mouth phenotype(s)
Current name Author & date Labeobarbus Intermediate Varicorhinus Notes Max. size References
1
10
20
Labeobarbus acuticeps (Matthes, 1959)
Labeobarbus acutirostris ( Bini, 1940) View in CoL
Labeobarbus aeneus View in CoL N2 ( Burchell, 1822) h & f Labeobarbus altianalis (Boulenger, 1900) View in CoL f Labeobarbus altipinnis ( Banister & Poll, 1973) View in CoL
Labeobarbus ansorgii (Boulenger, 1906) View in CoL
Labeobarbus aspius ( Boulenger, 1912) View in CoL
Labeobarbus axelrodi ( Getahun, Stiassny & Teugels,
2004)
Labeobarbus batesii (Boulenger, 1903)
Labeobarbus beso ( Rüppell, 1835) View in CoL
Labeobarbus boulengeri View in CoL N7 current paper
Labeobarbus brauni (Pellegrin, 1935)
Labeobarbus brevicephalus ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus brevispinis ( Holly, 1927) View in CoL
Labeobarbus bynni ( Forsskål, 1775) View in CoL f, ± f n Labeobarbus cardozoi ( Boulenger, 1912) View in CoL f s
Labeobarbus caudovittatus (Boulenger, 1902) View in CoL f
Labeobarbus clarkeae ( Banister, 1984) View in CoL
Labeobarbus claudinae View in CoL (De Vos & Thys van den f Audenaerde, 1990)
Labeobarbus codringtonii ( Boulenger, 1908) View in CoL h & f
Labeobarbus compiniei ( Sauvage, 1879) View in CoL h & f h
Labeobarbus crassibarbis ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus dainellii ( Bini, 1940) View in CoL f Labeobarbus dartevellei ( Poll, 1945) View in CoL
Labeobarbus dimidiatus ( Tweddle & Skelton, 1998) View in CoL
Labeobarbus ensifer ( Boulenger, 1910) View in CoL
Labeobarbus ensis ( Boulenger, 1910) View in CoL f s
Labeobarbus ethiopicus ( Zolezzi, 1939) View in CoL
Labeobarbus fasolt Pappenheim View in CoL (in Pappenheim & f h Boulenger, 1914)
a & ± a h 403 TL DeV&TvdA, 1990: 18
a h, r & n N1 411 FL Na&Si, 1997: 130
x rce N3 500 FL Sk, 2001: 169
± a l, r & n N4 540 SL Ec, 1992: 44
n h rce N5 338 SL Ba&Po, 1973: 82; Lé&Da, 1984: 336
n h 300 TL Bo, 1906a: 111; Lé&Da, 1984: 337
n s 420 TL Bo, 1912: 14; Lé&Da, 1984: 228; DeW&Te, 2007: 516–517
rce h 160 SL Ge et al., 2004: 160–161; Ge, 2007b: 542–543
± a h & n N6 435 TL Lé&Da, 1984: 230; Le, 2003: 367–368; DeW&Te, 2007: 520–521
rce n 360 TL Lé&Da, 1984: 337
rce l 160 TL Bo, 1910: 548; Lé&Da, 1984: 338
n s? rce s N8 195 SL; 245 TL Pe,1935: 402
n h N9 317 FL Na&Si, 1997: 131; Na&Si, 2000: 192
a s & n s 230 TL Lé&Da, 1984: 232; DeW&Te, 2007: 520–521
a & n N10 820 TL Lé&Da, 1984: 234
530 TL Bo, 1912: 12; Lé&Da, 1984: 238; DeW&Te, 2007: 526–527
a s & n rce (1 V. syn) N11 800 TL Lé&Da, 1984: 239; DeW&Te, 2007: 524 & 527; Ec, 1992: 45
pap h 161 SL Ba, 1984: 277
a h 234 SL; 300 TL DeV&TvdA, 1990: 7
n h N12 390 TL Sk, 2001: 173
N13 730 TL Sa, 1879: 102; Lé&Da, 1984:
241; DeW&Te, 2007: 528–529 a, r h & n N14 505 FL Na&Si, 1997: 132; Na&Si, 2000:
193 a, r h & n N15 490 FL Na&Si, 1997: 133 n h 117 TL Po, 1945: 299–300; Lé&Da,
1984: 241
rce h N16 245 SL Tw&Sk, 1998: 371; Sk, 2001:
174 pap s N17 195 TL Bo, 1910: 546; Lé&Da, 1984: 337 a s, r s & n s 140 TL Bo, 1910: 550; Lé&Da, 1984: 243 a & n N18 258 SL Ba, 1973: 41; Lé&Da, 1984: 243
500 TL Pa&Bo, 1914: 241; Lé&Da, 1984:
245
30
40
50
60
Labeobarbus fimbriatus ( Holly, 1926) View in CoL
Labeobarbus fritschii ( Günther, 1874) View in CoL
Labeobarbus gananensis ( Vinciguerra, 1895) View in CoL f h Labeobarbus gestetneri ( Banister & Bailey, 1979) View in CoL
Labeobarbus girardi ( Boulenger, 1910) View in CoL
Labeobarbus gorgorensis ( Bini, 1940) View in CoL
Labeobarbus gorguari ( Rüppell, 1835) View in CoL
Labeobarbus gruveli ( Pellegrin, 1911) View in CoL
Labeobarbus gulielmi ( Boulenger, 1910) View in CoL
Labeobarbus habereri ( Steindachner, 1912) View in CoL f h
Labeobarbus harterti ( Günther, 1901) View in CoL
Labeobarbus huloti (Banister, 1976) View in CoL f
Labeobarbus humphri (Banister, 1976) f
Labeobarbus intermedius ( Rüppell, 1835) View in CoL h & f Labeobarbus iphthimostoma ( Banister & Poll, 1973) View in CoL
Labeobarbus iturii ( Holly, 1929) View in CoL f? h Labeobarbus jaegeri ( Holly, 1930) View in CoL
Labeobarbus johnstonii (Boulenger, 1907) View in CoL f
Labeobarbus jubae ( Banister, 1984) View in CoL
Labeobarbus jubbi ( Poll, 1967) View in CoL f Labeobarbus kimberleyensis ( Gilchrist & Thompson, 1913) View in CoL
Labeobarbus lagensis ( Günther, 1868) View in CoL
Labeobarbus latirostris View in CoL N27 ( Keilhack, 1908)
Labeobarbus leleupanus (Matthes, 1959)
Labeobarbus longidorsalis (Pellegrin, 1935)
Labeobarbus longifilis (Pellegrin, 1935) View in CoL f Labeobarbus longissimus ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus lucius ( Boulenger, 1910) View in CoL
Labeobarbus lufupensis ( Banister & Bailey, 1979) View in CoL
Labeobarbus macroceps ( Fowler, 1936) View in CoL
Labeobarbus macrolepidotus (Pellegrin, 1928) View in CoL
Labeobarbus macrolepis ( Pfeffer, 1889
Labeobarbus macrophthalmus ( Bini, 1940)
Labeobarbus malacanthus ( Pappenheim, 1911) View in CoL
n s 213 TL
n s rce (2 V. syn) N19 180 TL a & n 176 SL n h 249 TL
n s 300 TL a, ± a h, r & N20 618 FL n ± a h, r & n N21 532 FL n h 280 TL
a s & n s 150 TL
162 TL
n s 285 TL
a & n h 282 SL
a h 214 SL
a, r? h & n rce (1 V. syn) N22 489 SL
rce h 181 SL
N23 365 TL
rce h 188 TL
a & n h rce (1 V. syn) N24 320 SL
rce h N25 135 SL
N26 166 SL; 225 TL a h 825 FL n h 230 SL/255 TL
n l 370 SL rce h 220 SL rce h 260 SL; 320 TL
a l 247 mm SL
r & n h N28 548 FL
n s 230 TL
n h 194 SL
n h 320 TL n s rce N29 540 TL n l 400 SL a, r h, & n N30 425 FL ± a h 150 TL
Ho, 1926: 157; Lé&Da, 1984: 338
Lé&Da, 1984: 246
Ba, 1973: 45; Lé & Da, 1984: 247 Ba& Bai, 1979: 218; Lé & Da, 1984: 247
Bo, 1910: 551; Lé&Da: 1984: 247 Na&Si, 1997: 134
Na&Si, 1997: 135
Pe, 1911: 185; Lé&Da, 1984: 247; Lé&Gu, 1990: 52–54; Le, 1990: 308–309
Bo, 1910: 551; Lé&Da, 1984: 248 Ho, 1927: 141; Lé&Da, 1984: 249; DeW&Te, 2007: 518–519 Lé&Da, 1984: 246
Ba, 1976b: 192; Lé&Da, 1984: 250
Ba, 1976b: 198; Lé&Da, 1984: 250
Lé&Da, 1984: 255
Ba&Po, 1973: 87; Lé&Da, 1984: 338
Ho, 1929: 35; Lé&Da, 1984: 255 Ho, 1930: 199; Lé&Da, 1984: 338; Ge, 2007b: 541–542 Ba&Cl, 1980: 505–506; Lé&Da, 1984: 256; Ec, 1992: 45
Ba, 1984: 273
Po, 1967: 177; Lé&Da, 1984: 257 Sk, 2001:168
Lé&Da, 1984: 259; Lé&Gu, 1990: 49; Le, 1990: 304; Le, 2003: 363–364
Ke, 1908: 166
Lé&Da, 1984: 338
Pe, 1935: 403; Lé&Da, 1984: 338 Ba, 1973: 76
Na&Si, 1997: 135
Bo, 1910: 553; Lé & Da, 1984: 262; DeW&Te, 2007: 514–515 Ba& Bai, 1979: 227; Lé & Da, 1984: 338
Fo, 1936: 283; Lé&Da, 1984: 263 Lé&Da, 1984: 339
Ec, 1992: 46
Na&Si, 1997: 136
Pa, 1911: 517; Lé&Da, 1984: 264; DeW&Te, 2007: 521–522 Mouth phenotype(s)
Current name Author & date Labeobarbus Intermediate Varicorhinus Notes Max. size References 70
80
90
Labeobarbus marequensis ( Smith, 1841) View in CoL h & f Labeobarbus mariae ( Holly, 1926) View in CoL
Labeobarbus maroccanus ( Günther, 1902) View in CoL
Labeobarbus matris ( Holly, 1928) View in CoL
Labeobarbus mawambi Pappenheim View in CoL (in Pappenheim &
Boulenger, 1914)
Labeobarbus mawambiensis ( Steindachner, 1911) View in CoL f s Labeobarbus mbami ( Holly, 1927) View in CoL
Labeobarbus megastoma ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus micronema (Boulenger, 1904) View in CoL
Labeobarbus mirabilis Pappenheim (in Pappenheim &
Boulenger, 1914)
Labeobarbus mungoensis ( Trewavas, 1974) View in CoL f
Labeobarbus nanningsi de Beaufort, 1933 f View in CoL h
Labeobarbus natalensis View in CoL N34 ( de Castelnau, 1861) h & f
Labeobarbus nedgia Rüppell, 1835 View in CoL ± h h & f
Labeobarbus nelspruitensis ( Gilchrist & Thompson, 1911) View in CoL
Labeobarbus nthuwa Tweddle & Skelton, 2008 View in CoL
Labeobarbus osseensis ( Nagelkerke & Sibbing, 2000) View in CoL f
Labeobarbus oxyrhynchus ( Pfeffer, 1889) View in CoL h & f
Labeobarbus pagenstecheri ( Fischer, 1884) View in CoL
Labeobarbus parawaldroni View in CoL (Lévêque, Thys van den h h & f Audenaerde & Traore, 1987)
Labeobarbus paucisquamatus (Pellegrin, 1935) View in CoL h Labeobarbus pellegrini ( Bertin & Estève, 1948) View in CoL
Labeobarbus petitjeani ( Daget, 1962) View in CoL
Labeobarbus platydorsus ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus platyrhinus (Boulenger, 1900) View in CoL
Labeobarbus platystomus Pappenheim (in Pappenheim &
Boulenger, 1914)
Labeobarbus pojeri ( Poll, 1944) View in CoL
Labeobarbus polylepis (Boulenger, 1907) View in CoL x? Labeobarbus progenys (Boulenger, 1903) View in CoL
Labeobarbus pungweensis ( Jubb, 1959) View in CoL
N48
Labeobarbus reinii ( Günther, 1874) View in CoL f s Labeobarbus rhinoceros View in CoL N49 Copley, 1938
Labeobarbus rhinophorus ( Boulenger, 1910) View in CoL
Labeobarbus robertsi ( Banister, 1984) View in CoL
a? h, r & n rce (2 V. syn) N31 470 TL Sk, 2001: 172
n? s rce s (V. -like) N32 300 SL Ho, 1926: 156; Lé&Da, 1984:
339; Ge, 2007b: 543–544
rce s 400 TL Lé&Da, 1984: 339
a h 322 TL Ho, 1928: 4
n h 150 TL Lé&Da, 1984: 268
± a s & a s 150 TL Lé&Da, 1984: 268 n h 230 SL DeW&Te, 2007: 524–525 a h, r & n N33 824 FL Na&Si, 1997: 137; Na&Si, 2000: 197 n s 340 TL Lé&Da, 1984: 268; DeW&Te, 2007: 518–519 r h 353 SL Ba, 1973: 92; Lé&Da, 1984: 270
a h & n 179 SL Lé&Da, 1984: 271; DeW&Te, 2007: 522–523
320 TL Po, 1967: 157; Lé&Da, 1984: 271
a & n rce N35 638 TL Sk, 2001: 170
N36 707 FL Na&Si, 1997: 138
rce s N37 320 TL Sk, 2001: 175
a h & n rce N38 236 SL Tw&Sk, 2008: 29
n h N39 264 SL Na&Si, 2000: 190
a, r & n h rce (1 V. & 1 V. -like syn) N40 400 SL Ec, 1992: 46
± a l rce (V. - like) N41 350 SL Ec, 1992: 45
N42 230 SL Lé et al., 1987: 347; Lé&Gu, 1990: 48; Lé, 1990: 303; Lé, 2003: 363–364
a l & r N43 248 SL Ba, 1973: 106; Lé&Da, 1984: 279
rce? h N44 212 TL Pe, 1932: 959; Lé&Da, 1984: 339
a s, r s & n s N45 175 SL Lé&Gu, 1990: 53
a, r, ± r h & n N46 635 FL Na&Si, 1997: 139; Na&Si, 2000: 199
r h 400 SL Ec, 1992: 45
rce s 210 TL Lé&Da, 1984: 339
a h & n 180 TL Po, 1944: 6 a h rce? N47 585 TL Lé&Da, 1984: 282 n h 180 TL Lé&Da, 1984: 282; Le, 2003:
366–367; DeW&Te, 2007:
516–517
rce h 180 SL Sk, 2001: 176 a s 555 TL Pe, 1921: 136; Lé&Da, 1984: 285 n l 295 TL Ba, 1973: 83 n s 150 TL Lé&Da, 1984: 285 pap h 220 SL WaLu, 2010: 142
Labeobarbus rocadasi ( Boulenger, 1910) View in CoL
± a s, r s & n s 350 TL
Labeobarbus rosae ( Boulenger, 1910) View in CoL n? s rce s (V. - like) N50 95 TL
100 Labeobarbus roylii ( Boulenger, 1912) View in CoL h s & f s 550 TL
Labeobarbus ruandae Pappenheim View in CoL (in Pappenheim & rce h 150 TL Boulenger, 1914)
Labeobarbus ruasae Pappenheim (in Pappenheim & ± f s ± a s 430 SL; Boulenger, 1914) 495 TL
Labeobarbus ruwenzorii ( Pellegrin, 1909) View in CoL rce s N51 231 TL
Labeobarbus sacratus ( Daget, 1963) View in CoL f a, r s & n s N52 256 SL
110
Labeobarbus sandersi ( Boulenger, 1912) View in CoL
Labeobarbus seeberi View in CoL N54 ( Gilchrist & Thompson, 1913) h & f Labeobarbus semireticulatus ( Pellegrin, 1924) View in CoL
Labeobarbus somereni (Boulenger, 1911) View in CoL
Labeobarbus stappersii ( Boulenger, 1915) View in CoL f Labeobarbus steindachneri ( Boulenger, 1910) View in CoL
Labeobarbus stenostoma ( Boulenger, 1910) View in CoL
Labeobarbus surkis ( Rüppell, 1835) View in CoL
Labeobarbus tornieri ( Steindachner, 1906) View in CoL
Labeobarbus trachypterus ( Boulenger, 1915) View in CoL
n rce l N53 370 TL a s, r s & n s rce (V. -like) N55 987 TL n? s rce s N56 128 SL;
158 TL ± n h 400 SL a & n h N57 594 SL
rce s 330 SL
rce h 105 TL r & n h N58 430 FL
rce h 184 SL n h rce (1 V. syn) N59 239 SL
Labeobarbus tropidolepis (Boulenger, 1900)
Labeobarbus truttiformis ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus tsanensis ( Nagelkerke & Sibbing, 1997) View in CoL
Labeobarbus upembensis ( Banister & Bailey, 1979) View in CoL
Labeobarbus urotaenia View in CoL N63 ( Boulenger, 1913) f s 120 Labeobarbus varicostoma ( Boulenger, 1910) View in CoL
Labeobarbus versluysii ( Holly, 1929) View in CoL
Labeobarbus werneri ( Holly, 1929) View in CoL
Labeobarbus wittei ( Banister & Poll, 1973) View in CoL
Labeobarbus wurtzi ( Pellegrin, 1908) View in CoL
125 Labeobarbus xyrocheilus ( Tweddle & Skelton, 1998) View in CoL
Hybrids and possible hybrids
Labeobarbus alluaudi ( Pellegrin, 1909) View in CoL
Labeobarbus microbarbis ( David & Poll, 1937) View in CoL
Labeobarbus microterolepis (Boulenger, 1902) View in CoL
Others
Acapoeta tanganicae (Boulenger, 1900)
Sanagia velifera Holly, 1926 View in CoL
a, r l & n N60 850 SL
n h N61 442 FL
a h, r & n N62 394 FL
rce h 203 SL
a s 50 TL
pap h 170 TL a s N64 111 TL
rce?s N65 145 TL
n h rce N66 375 TL
n h rce N67 285 SL rce h N68 178 SL
n s 198 SL
n h 230 SL; 270 TL ± n h 118 SL
rce l N69 610 TL rce s (V. - like) N70 158 SL; 190 T
Lé&Da, 1984: 285; DeW&Te, 2007: 526–527
Lé&Da, 1984: 286
DeW&Te, 2007: 523–524
Lé&Da, 1984: 340
DeV&TvdA, 1990: 19
Lé&Da, 1984: 340
Lé&Gu, 1990: 56; Le, 1990: 307; Le, 2003: 367–368
Bo, 1912: 12; Lé&Da, 1984: 340 Sk, 2001: 171
Pe, 1924: 287; Lé&Da, 1984: 340; Ge, 2007b: 545–546
Ec, 1992: 46
Ba, 1973: 119; Lé&Da, 1984: 288 Ge, 2007b: 546–547
Bo, 1910: 546; Lé&Da, 1984: 341 Na&Si, 1997: 140
Ge, 2007b: 547–548
Ba, 1973: 120; Lé&Da, 1984: 291–292
Ec, 1992: 44
Na&Si, 1997: 141; Na&Si, 2000: 200
Na&Si, 1997: 142; Na&Si, 2000: 202
Ba& Bai, 1979: 229; Lé & Da, 1984: 341
Lé&Da, 1984: 295
Bo, 1910: 547; Lé&Da, 1984: 341 Ho, 1929: 33; DeW&Te, 2007: 528–529
Ge, 2007b: 548–549
Ba&Po, 1973: 91; Lé&Da, 1984: 341
Lé&Gu, 1990: 61
Tw&Sk, 1998: 379; Sk, 2001: 370
Ba, 1972: 276; Ba, 1973: 9; Lé&Da, 1984
DeV&TvdA, 1990: 20–21
Ba, 1973: 89; Lé&Da, 1984: 268
Lé&Da, 1984: 217
Lé&Da, 1984: 336; DeW, 2007: 536–537
Nominal species representing hybrid phenotypes, or suspected to be doing so, according to the literature cited, have been listed separately at the end of the table. Also both Acapoeta and Sanagia species have been added at the end as a matter of completeness (i.e. total +2). Unjustified revalidations, however, i.e. the reuse of formally synonymized species names without any explicit justification with regards to this decision, have not been followed. The same holds true for unsubstantiated proposals of possible new synonyms/ies. All species names are organized in alphabetic order.
For each species the following information is provided: current species name (Current name); author(s) and date of the original description (Author & date); maximum size (Max. size) reported (FL, fork length; SL, standard length; TL, total length; all in mm); documented mouth phenotype(s) [Mouth phenotype(s)], and as such possible mouth polymorphism; and references (References) used for the current compilation of the tabulated information with regards to the maximum reported size. Additional information has been provided under Notes, referred to in the table with superscript N, and followed by the number of the particular note. The notes themselves are to be found below the table.
Mouth phenotype(s) as well as possible mouth phenotype polymorphism have been tabulated for each of the valid species to get a first pan-African overview of this peculiar phenomenon. For this, the observed mouth phenotype diversity has been subdivided into three major categories, i.e. the Labeobarbus versus Varicorhinus mouth phenotype as the two extreme mouth phenotype categories and with a third, the intermediate category, in between these. The typical Labeobarbus mouth phenotype has been defined as characterized by the presence of, at least, a free mental lobe, and has been further subdivided according to the presence/absence of hypertrophied lips (i.e. the anteromedian part of the upper lip forming a flap-like extension clearly covering the anteromedian part of the snout). The typical Varicorhinus mouth phenotype has been restricted to species with the presence of a clear cornified cutting edge on the lower jaw. Finally, specimens with an attached or rudimentary (i.e. no real lobe, but only a discontinuous suture line demarcating a lobe-like structure) mental lobe, or without a mental lobe, have been tabulated as intermediates. Priority has been given to tabulate the mouth phenotype(s) of the type (s) with special attention in differentiating the mouth phenotype(s) as found in the name-bearing type (s), which is/are the only one(s) with nomenclatural standing (see ICZN, 1999: articles 72.1.2. and 72.1.3.). Whenever available, and based on sound, reliable, revisionary evidence [see Nagelkerke & Sibbing (1997, 2000) for the Lake Tana ( Ethiopia) species and Skelton (2001) for the Southern African species], mouth phenotype polymorphism as reported for the species as a whole, i.e. including non-types, has been included.
The abbreviations used to characterize these mouth phenotypes are as follows: a, attached [equals continuous lower lip development (LLD) of Nagelkerke & Sibbing (2000: fig. 2)]; f, free; h, hypertrophied; n, no [equals interrupted LLD of Nagelkerke & Sibbing (2000: fig. 2)]; and r, rudimentary mental lobe. A few Labeobarbus species have pappilae (pap) on the lower as well as the upper jaw instead. These have explicitely been reported as such under the intermediate mouth phenotype, although, in these cases, this does not imply the intermediacy of this character state. A question mark (?) has been used when, unfortunately: (1) the detailed mouth phenotype remains undocumented [i.e. not stipulated in the literature and the name-bearing/nominal type(s) are lost or have not been examined]; (2) the present character state is doubtful, this most often because of damage or due to the actual state of preservation of the type(s) (see Notes). Whenever personal observation of the mouth phenotype character state on the name-bearing/nominal type(s) of the valid species has been possible, reference is made to these results. The character state as found in the name-bearing/nominal type(s) is made explicit by using bold font for the mouth phenotype(s). Details on the nominal type(s) have been added in subscript: h, holotype; l, lectotype; s, syntypes. An ‘x’ has been used when the literature refers to the existence of one of the three major mouth phenotypes without, however, providing the necessary details to add more information. As a general rule, we have refrained from explicitely tabulating mouth phenotype(s) as found in junior synonym(s); however, when former Varicorhinus species have been synonymized with a valid Labeobarbus mouth phenotype species, this has explicitly been stipulated as follows: V. syn or V.-like syn, Varicorhinus or Varicorhinus -like mouth phenotype synonym(s), preceeded by the number of such synonyms. A Varicorhinus -like mouth phenotype refers to the fact that it has a typical real cutting edge (rce), but nevertheless differs in other respects from the typical Varicorhinus mouth phenotype, which has been documented in the notes. Indeed, in the typical Varicorhinus mouth phenotype, the fleshly lips on the lateral sides of the lower jaw are poorly developed (see Boulenger, 1909: plate 33 for V. beso ), i.e. covering approximately only half of the lateral sides of the lower jaw, but very often much less (one-third or even only one-quarter, as in V. beso itself; with important intraspecific variation in some species as currently delimited). Instead, in the Varicorhinus -like mouth phenotype, the fleshly lips on the lateral sides of the lower jaw are obviously more developed, i.e. covering approximetely two-thirds or more of the lateral sides of the lower jaw (e.g. L. rosae ; Sanagia velifera ). Nevertheless, considering: (1) the very poor development of the lips in V. beso (see above); (2) the important inter- and sometimes even intraspecific variation, this character is certainly in need of further attention (see text). In addition, whenever relevant with regards to the tabulated overall intraspecific mouth phenotype variation, reference is also made to the mouth phenotype of the name-bearing/nominal type(s) of other junior synonyms (i.e. non Varicorhinus nominal species) in the notes. The junior synonyms examined have been listed in the notes, grouped according to their mouth phenotype, and listed from the Labeobarbus through the intermediate to the Varicorhinus mouth phenotype.
Abbreviations of the author names for the references cited are as follows: Ba, Banister; Ba&Cl, Banister & Clarke; Ba& Bai, Banister & Bailey; Ba&Po, Banister & Poll; Bo, Boulenger; DeV&TvdA, De Vos & Thys van den Audernaerde; DeW, De Weirdt; DeW&Te, De Weirdt & Teugels; Fo, Fowler; Ec, Eccles; Ge, Getahun; Ho, Holly; Ke, Keilhack; Lé, Lévêque; Lé&Da, Lévêque & Daget; Lé&Gu, Lévêque & Guegan; Na&Si, Nagelkerke & Sibbing; Pa, Pappenheim; Pe, Pellegrin; Po, Poll; Sa, Sauvage; Sk, Skelton; Tw&Sk, Tweddle & Skelton; and WaLu, Wamuini Lunkayilakio. N1. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5, see also page 130)]. N2. No type ( Lévêque & Daget, 1984: 220). Burchell (1822: 280) most probably had several specimens before him when describing the species, as he provided some variation for the number of pectoral fin rays. N3. Labeobarbus to Varicorhinus mouth phenotype specimens present (see Skelton, 2001: 169). Synonyms: holotype of Labeobarbus gilchristi (Boulenger, 1911) (BMNH 1909.12.8.1: 149 mm SL) with a typical Labeobarbus hypertrophied mouth phenotype (h) and holotype of Labeobarbus mentalis ( Gilchrist & Thompson, 1913) (SAIAB 134770: 257 mm SL) with a typical Labeobarbus free mental lobe (f). N4. Labeobarbus + intermediate mouth phenotype polymorphism (see Worthington, 1929: 432, fig. 3; 1932: 126; fig. 1). Synonyms: one of both syntypes of Labeobarbus lobogenys (Boulenger, 1906) [BMNH 1906.5.30.117–221 (two instead of five syntypes): 218–223 mm SL] with a typical Labeobarbus free mental lobe (f); at least some of the syntypes of Labeobarbus kiogae ( Worthington, 1929) [BMNH 1929.1.24.105–108 (seven instead of four syntypes): 185–417 mm SL] with an intermediate mouth phenotype (r); and holotype of Labeobarbus eduardianus (Boulenger, 1901) (BMNH 1906.9.7.41: 379 mm SL) and syntypes of Labeobarbus fergusonii (Boulenger, 1901) (BMNH 1906.9.7.42–43: 223–224 mm SL) with an intermediate mouth phenotype (n). N5. Although reported by Banister & Poll (1973: 84), the holotype (MRAC 179729: 266 mm SL) lacks an rce (see also illustration in Banister & Poll, 1973: 85, fig. 2); however, two paratypes have one (MRAC 179730, 97 mm SL; MRAC 179735, 338 mm SL). N6. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Thys van den Audenaerde, 1967: 35, fig. 12)]. N7. Replacement name for Varicorhinus latirostris Boulenger, 1910 (now Labeobarbus ) (see text and annotated checklist 1). N8. MNHN 1935-0066 syntype (171 mm SL) with an rce; however, MRAC 42933 syntype (195 mm SL) actually without an rce, possibly lost over time. N9. Intermediate mouth phenotype [interrupted (i.e. no lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 131)]. N10. Synonyms: holotype of Labeobarbus seguensis ( Pellegrin, 1925) (MNHN 1925-0193, 495 mm SL) with a typical Labeobarbus free mental lobe (f); holotype of Labeobarbus occidentalis (Boulenger, 1911) (BMNH 1909.3.3.14, 74 mm SL) with an intermediate mouth phenotype (a); and holotype of Labeobarbus meneliki ( Pellegrin, 1905) (MNHN 1905- 0275, 191 mm SL) with an intermediate mouth phenotype (n). N11. Synonyms: holotype of Labeobarbus euchilus (Boulenger, 1920) (BMNH 1919.7.24.7, 66 mm SL) with a typical Labeobarbus free mental lobe (f); syntypes of Labeobarbus miochilus (Boulenger, 1920) (BMNH 1919.4.24.8–9, 70– 71 mm SL; MRAC 6992, 61 mm SL) with an intermediate mouth phenotype (n); and syntypes of Varicorhinus stappersii Boulenger, 1917 (now Labeobarbus ) [BMNH 1920.5.25.36–37, 186–244 SL; MRAC 14197, 250 mm SL; and 14222, 175– 172 mm SL (two instead of one syntype)], with a typical Varicorhinus mouth phenotype rce. N12. Variable Labeobarbus mouth phenotype polymorphism present, with frequently thickened lips (rubber lips) (see Skelton, 2001: 173). Synonyms: holotype of Labeobarbus altidorsalis ( Boulenger, 1908) (BMNH 1908.11.6.26, 3212 mm SL) and syntypes of Labeobarbus chilotes ( Boulenger, 1908) (BMNH 1908.11.6.24–25, 125– 199 mm SL), with a typical Labeobarbus free mental lobe (f). N13. Labeobarbus mouth phenotype polymorphism. Synonyms: holotype of Labeobarbus labiatomimus ( Pellegrin, 1914) (MNHN 1886-0395, 240 mm SL), with a typical Labeobarbus hypertrophied mouth phenotype (h). N14. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997, table 5.5., see also page 132)]. N15. Intermediate + Labeobarbus mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe), or small lobe (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 133)]. N16. See also Tweddle & Skelton (1998: 372). N17. Boulenger (1910: 546, 1916a: 214) reports a ‘cutting-edge’ for (some?) syntypes, but there is none. N18. Holotype (MCZR) not seen. Based on three BMNH, non-type, specimens (BMNH 1971.7.12.1–3, 240– 258 mm SL; see Banister, 1973: 41). N19. Synonyms: syntypes of Labeobarbus rothschildi ( Günther, 1901) (BMNH 1901.4.26.6–7, 127– 134 mm SL), Labeobarbus riggenbachi ( Günther, 1902) (BMNH 1902.7.28.19, 115 mm SL and 1902.7.28:20–21, 136– 137 mm SL) and Labeobarbus paytonii (Boulenger, 1911) [BMNH 1903.10.29.17–20, 50– 116 mm SL (seven instead of four syntypes)], all with intermediate mouth phenotypes (n); syntypes of Capoeta atlantica Boulenger, 1902 (BMNH 1902.1.4.18–19, 99– 112 mm SL) and one of both syntypes of Capoeta waldoi Boulenger, 1902 [BMNH 1902.1.4.16–17: 129–140 mm SL (probably lost over time in the largest of both syntypes)] have a Varicorhinus mouth phenotype with a typical rce; however, the rce is very fine, does not cover the entire anterior width of the lower jaw, and the fleshy lips of the sides of the lower jaw are better developed than in the typical Varicorhinus mouth phenotype. N20. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 134)]. N21. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 135)]. N22. Synonyms: most of the syntypes of Labeobarbus gudaricus (Boulenger, 1906) [BMNH 1908.1.20.131–132, 140– 218 mm SL (six instead of two specimens) except BMNH 1908.1.20.133, 234 mm SL, which has an intermediate mouth phenotype (a)], with a typical Labeobarbus free mental lobe (f) and sometimes even a hypertrophied mouth phenotype (h); as reported by Nagelkerke & Sibbing (1997: 121), two syntypes of Labeobarbus leptosoma (Boulenger, 1902) (BMNH 1902.12.13.300–302), two of the syntypes have a continuous lower lip (f; 138 and 205 mm SL), whereas it is indeed interrupted in the third one (r, 208 mm SL) as in Barbus intermedius ; although both have originally been described as Barbus species , at least one of the syntypes of Labeobarbus macmillani (Boulenger, 1906) [BMNH 1908.1.20.103–106, 141– 192 mm SL (i.e. the largest syntype; cover seems lost over time in the other syntypes) and 1937.4.20.68, 176 mm SL] and most of the syntypes of Labeobarbus plagiostomus (Boulenger, 1902) [BMNH 1902.12.13.271–272, 176– 193 mm SL (i.e. the smallest of both syntypes; cover seems lost over time in the other syntype) and 1902.12.13.273, 176 mm SL], with a typical Varicorhinus -like rce, except for one of the syntypes of the latter species, where it seem lost through time; and holotype of Capoeta bingeri Pellegrin, 1905 (MNHN 1905-0252), also with a typical Varicorhinus mouth phenotype, i.e. an rce. N23. Holotype (NMW) apparently lost. N24. Intermediate and Varicorhinus mouth phenotype polymorphism present (see Banister & Clarke, 1980: 500–504). Synonyms: at least one of the syntypes of Labeobarbus globiceps ( Worthington, 1933) (BMNH 1932.11.15.275– 282, 235 mm SL) with a typical Labeobarbus free mental lobe (f); and one of the two syntypes of Labeobarbus njassae ( Keilhack, 1908) [ZMB 18163, 107 mm SL, and ZMB 18164, 74 mm SL (cover seems lost over time in the smallest of both syntypes)] and the syntypes of Labeobarbus nyasensis ( Worthington, 1933) (BMNH 1932.11.15.387– 392, 207 mm SL; and BMNH 1932.11.15.393–395, 202– 298 mm SL) – the latter a former Varicorhinus species – with a typical Varicorhinus rce. N25. See also Levin et al. (2013: 401, fig. 1b). N26. Holotype (Museo do Dundo: MD 1078) not seen. Statement based on a re-examination of paratypes (MRAC 161065 and 161066, 63 and 85 mm SL, respectively) only. N27. Previously identified as a junior synonym of Labeobarbus johnstonii (Boulenger, 1907) , but to be considered a valid species. Species revalidated in the present paper (see text and annotated checklist 1). N28. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or ± continuous (i.e. rudimentary lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 135)]. N29. Whereas all syntypes of Labeobarbus macrolepidotus lack an rce and therefore have an intermediate mouth phenotype (n), the specimens of the Inkisi Basin all have a typical Varicorhinus mouth phenotype, i.e. an rce. N30. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 136)]. N31. Synonyms: two syntypes of Labeobarbus zambezensis Peters, 1852 (ZMB 3246, 80 and 93 mm SL), with a typical Labeobarbus mouth phenotype (f & h, respectively) and another (NMW 49730: 77 mm SL) with an intermediate mouth phenotype (n); holotype of Labeobarbus brucii (Boulenger, 1907) (BMNH 1907.3.15.34, 131 mm SL) with a typical Labeobarbus free mental lobe (f); at least one of the syntypes of Labeobarbus inermis ( Peters, 1852) (ZMB 4736, syntype of 58 mm SL) with an intermediate mouth phenotype (r); holotype of Varicorhinus brucii Boulenger, 1907 (now Labeobarbus ) (BMNH 1907.3.15.37, 149 mm SL) [with replacement name Barbus oliphanti Keilhack, 1910 (now Labeobarbus )] and holotype of Varicorhinus nasutus Gilchrist & Thompson, 1911 (now Labeobarbus ) (SAIAB 134736, ± 367 mm SL), both with a typical Varicorhinus rce; and holotype of Labeobarbus sector (Boulenger, 1907) also with a typical Varicorhinus rce (BMNH 1907.3.15.35, 116 mm SL). N32. Originally described as a Varicorhinus species by Holly (1926); however, without special reference to the rce in the original description. In two of the three syntypes the cornified cover is entirely absent (NMW 7221, 128 mm SL, and NMW 7222-223, 88 mm SL), and most probably lost over time. Only in the third syntype (NMW 7222-223, 107 mm SL) does a faint rce seem to remain; however, all syntypes have well-developed fleshy lips on the lateral sides of the lower jaw, not at all resembling the almost entire absence of lips as found in the typical Varicorhinus mouth phenotype. N33. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 137)]. N34. Apparently no type (see Lévêque & Daget, 1984: 271). N35. Labeobarbus and Varicorhinus mouth phenotype polymorphism present (see Crass, 1964: 53, fig. 7; Skelton, 2001: 170). Synonyms: holotype of Labeobarbus lobochilus (Boulenger, 1911) (BMNH 1908.12.28.96, 143 mm SL) with a hypertrophied typical Labeobarbus free mental lobe (h); holotype of Labeobarbus mfongosi ( Gilchrist & Thompson, 1913) (SAIAB 135057, 200 mm SL) with a typical Labeobarbus free mental lobe (f); syntypes of Labeobarbus bowkeri (Boulenger, 1902) (BMNH 1862.8.28.3–8, 179– 222 mm SL; BMNH 1874.3.5.1–2, 124– 128 mm SL; BMNH 1894.7.10.4, 101 mm SL) with an intermediate mouth phenotype (a or n) or a typical, although small, Labeobarbus free mental lobe (f); holotype of Labeobarbus robinsoni ( Gilchrist & Thompson, 1913) (SAIAB 135055, 164 mm SL) with an intermediate mouth phenotype (a); and holotype of Labeobarbus zuluensis ( Gilchrist & Thompson, 1913) (SAIAB 134939, 325 mm SL) with an intermediate mouth phenotype (n). N36. Labeobarbus mouth polymorphism [lobe: small or large (see Nagelkerke & Sibbing, 1997: table 5.5, see also page 138)]. N37. Groenewald (1958: 277) and others. N38. Intermediate and Varicorhinus -like mouth phenotype polymorphism present (see Tweddle & Skelton, 2008: 30). Indeed, one of the paratypes (SAIAB 51928, 94 mm SL) has an rce. N39. Intermediate + Labeobarbus mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe), or small lobe (see Nagelkerke & Sibbing, 2000: table 3)]. N40. Synonyms: holotype of Labeobarbus labiatus (Boulenger, 1902) (BMNH 1902.5.26.37, 236 mm SL) with a hypertrophied lobe mouth phenotype (h); at least some of the syntypes of Labeobarbus tanensis ( Günther, 1894) (BMNH 1893.12.2.24–29, 191– 286 mm SL) with a typical, although small, Labeobarbus free mental lobe (f); syntypes of Labeobarbus hindii (Boulenger, 1902) (BMNH 1902.5.26.25–28, 84– 202 mm SL) with an intermediate mouth phenotype (a, r, or n); and holotype of Barbus (Capoeta) babaulti Pellegrin, 1926 (MNHN 1926-0285, 290 mm SL) and both syntypes of Barbus (Capoeta) perplexicans Boulenger, 1902 (BMNH 1902.5.26.35–36, 115– 144 mm SL), both with a Varicorhinus -like mouth phenotype, i.e. with well-developed fleshy lips on the lateral sides of the lower jaw (and even more so in the latter of both nominal species), not at all resembling the almost entire absence of lips as found in the typical Varicorhinus mouth phenotype. N41. We follow Ladiges et al. (1958) with regards to the lectotype designation for Labeobarbus pagenstecheri (see annnoated checklist 1): ‘The mouth is sub-terminal with a sharp [ Varicorhinus rce] edge to the lower jaw in the paralectotype [ZMH H 342, 210 mm SL] but rubber lips are developed in the... lectotype [ZMH H 341, 319 mm SL]′ (see Banister, 1973: 101; see also Seegers, 2008: 155); however, the paralectotype has well-developed fleshy lips on the lateral sides of the lower jaw, not at all resembling the almost entire absence of lips as found in the typical Varicorhinus mouth phenotype. N42. Labeobarbus mouth polymorphism (see Lévêque et al., 1987: 348, fig. 1). N43. Holotype of Labeobarbus altianalis lobogenysoides (Pellegrin, 1935) (MNHN 1935-0154) with a hypertrophied lobe mouth phenotype (h). N44. Lower jaw not bearing a cornified cover anymore; the cover seems to have fallen off. As such, the presence of an rce cannot be fully confirmed for this species. No details provided by Pellegrin (1932) in the original description. N45. see also Daget (1962: 78, fig. 16). N46. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 139)]. N47. Gaigher (1975: 162) reports the presence of Varicorhinus mouth phenotype in Labeobarbus polylepis . Skelton (2001: 170) reported that the lips are variable, but without any further details. N48. See also Jubb (1959: 308). N49. Substitute name for Barbus mariae Holly, 1929 , now Labeobarbus mariae ( Holly, 1929) (see text and annotated checklist 1). With the kind help of Helmut Wellendorf (2013), former curator of fishes at the NMW, the holotype of Barbus matris (NMW 8000, 243 mm SL) as well as both the lectotype [NMW 96652 (ex. 6562), 225 mm SL] and paralectotype of Barbus mariae [NMW 96653 (ex. 6562), 206 mm SL], the latter two designated but not seen by Banister (1973: 93–94), have all been traced (see text and annotated checklist 1). N50. An rce present in the two largest syntypes (79 and 80 mm SL). Nevertheless, absent in the smallest syntype (44 mm SL), but possibly lost over time. Despite the presence of a typical Varicorhinus rce in both the largest syntypes, they both also have welldeveloped fleshy lips on the lateral sides of the lower jaw, not at all resembling the almost entire absence of lips as found in the typical Varicorhinus mouth phenotype species. N51. All syntypes have an rce (MNHN 1909-0583-0585, 63–68 mm SL). According to Banister (1972: 121), present with increasing size, and absent in a specimen of 40 mm SL only (no further details provided). Note, however, that Banister (1972: 271) himself states that he studied specimens from 57 to 231 mm SL only. N52. Labeobarbus and intermediate mouth phenotype polymorphism [see Lévêque & Guégan, 1990: 54, fig. 14 (syntypes) & 55, fig. 15 (specimen: from Daget, 1962: 76, fig. 15 as Barbus gruveli )]. N53. An rce is present in the lectotype but absent in two paralectotypes, i.e. the smallest of the BMNH 1912.4.1.334– 336 paralectotypes (149 mm SL), as well as in the MRAC 1526 paralectotype (173 mm SL). N54. As demonstrated by E. Vreven, P.H. Skelton & E.R. Schwartz (unpubl. data), Barbus capensis is not a Labeobarbus but instead a senior synonym of Barbus andrewi . Therefore, Barbus seeberi , previously a junior synonym of Barbus capensis , and indeed a yellowfish or Labeobarbus , becomes the valid species name for the clanwilliam yellowfish (see also text and annotated checklist 1). N55. Labeobarbus mouth phenotype polymorphism (see Skelton, 2001: 171). In addition, specimens with an rce have been found (SAIAB 54113, 134 mm SL; SAIAB 54688, 176 mm SL); however, both have well-developed fleshy lips on the lateral sides of the lower jaw, not at all resembling the almost entire absence of lips as found in the typical Varicorhinus mouth phenotype. In addition, specimens with a typical Labeobarbus free mental lobe (f) (SAIAB 58362: 179 mm SL) and hypertrophied lips have also been found (h) (SAIAB 58418, 141 mm SL; SAIAB 65536, 101 mm SL). N56. One syntype with (MNHN 1924-0052, 56 mm SL) and one actually without (MNHN 1924-0052, 117 mm SL) an rce; however, the rce was most probably lost over time in the latter syntype. N57. Synonyms: holotype of Labeobarbus moeruensis ( Pellegrin, 1922) (MRAC 14765, 578 mm SL) and holotype of Labeobarbus oxycephalus ( Boulenger, 1915) (MRAC 14233, 266 mm SL), both with a typical Labeobarubs free mental lobe (f); holotype of Labeobarbus curtus ( Boulenger, 1915) (MRAC 17172, 233 mm SL) with an intermediate mouth phenotype (a). N58. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or ± continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 132)]. N59. Synonyms: Varicorhinus bredoi Poll, 1948 (now Labeobarbus ) [holotype IRSNB 76(1), 130 mm SL; paratypes IRSNB 77(2), 60–67 mm SL], with an rce (see Poll, 1948: 9–10, fig. 1–2), although, as mentioned by Poll (1948) himself, clearly absent in the smallest of both paratypes, which has an intermediate mouth phenotype (n). N60. Synonyms: Varicorhinus chapini Nichols & La Monte, 1950 (now Labeobarbus ) [holotype: AMNH 18785] with ‘... a narrower, less specilialized mouth than is usual in the genus [ Varicorhinus ]...’ ( Nichols & La Monte, 1950: 175). Indeed, holotype with ‘... lower jaw with a sharp, firm but not cartilaginous, edge,...’ ( Nichols & La Monte, 1950: 175), i.e. without the typical Varicorhinus mouth cutting edge, but with an attached lobe (a), and therefore with an intermediate mouth phenotype instead. N61. Intermediate mouth phenotype [interrupted (i.e. no lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 141)]. N62. Intermediate mouth phenotype polymorphism [interrupted (i.e. no lobe) or continuous (i.e. attached lobe) (see Nagelkerke & Sibbing, 1997: table 5.5., see also page 142)]. N63. Based on pers. observ. (E. Vreven 2013, pers. observ.) of the syntypes (BMNH 1912.12.6.5, 38 mm SL; MRAC 1191, 36 mm SL; MRAC 1192, 41 mm SL), clearly described based on three juvenile Labeobarbus syntype specimens: i.e. the one MRAC syntype with a typical Labeobarbus free mental lobe (f) (MRAC 1192) and the BMNH and the other MRAC syntype with an intermediate mouth phenotype (a). N64. See also Holly (1930: plate 1, fig. 8a), as illustrated for one of the syntypes. N65. Syntype (NMW 13948, 59 mm SL) seemingly without an rce. Most probably lost over time as Holly (1929: 32), in his original description of the species, refers to an rce: ‘Der unterkiefer trägt eine dünne Hornschneide;...’. Additional syntype (NMW 13949) not found (H. Wellendorf, pers. comm., 2000; E. Vreven, pers. observ., 2013). N66. Banister & Poll (1973: 91) reported the presence of a cornified cover, but not the presence of an rce. Indeed, the holotype lacks the rce, which conforms to the illustration provided by Banister & Poll (1973: 93, fig. 10); however, one paratype clearly has one (MRAC 179742, 385 mm SL). N67. The holotype of Labeobarbus wurtzi lacks an rce. Instead, a cornified cover, although damaged, and without an rce, is still present on the lower jaw; however, specimens with an rce have been found in collections (MNHN and MRAC) and, most probably, represent a species new to science (see text). N68. See Tweddle & Skelton (1998: 377). N69. According to Banister (1976a: 177), the anterior edge of the lower jaw is gently curved and a cornified cover is altogether lacking in fishes less than 60 mm SL. N70. Although an rce is present, it covers, approximately, only half of the anterior width of the lower jaw (see also Banister & Thys van den Audenaerde, 1973: 184, fig. 2) instead of almost the entire anterior width, resulting in a spoon-like rather than a spade-like cutting edge, as found in Labeobarbus spp. with a Varicorhinus , or a Varicorhinus -like, mouth phenotype. In addition, both syntypes have well-developed fleshy lips on the lateral sides of the lower jaw, not at all resembling the almost entire absence of lips found in the typical Varicorhinus mouth phenotype.
Furthermore, Varicorhinus capoetoides Pellegrin, 1938 has not been retained in our list either. Two characters, and especially the first one, make the placement of this species in the genus Varicorhinus , now Labeobarbus s.l., untenable: (1) the last dorsal fin ray is a long, bony, and serrated spine (see also Pellegrin, 1938) not known to occur in any other African Varicorhinus , now Labeobarbus s.l., species; (2) the high number of lateral line scales, i.e. 87 (see Pellegrin, 1938), a number only comparable with that found in Acapoeta tanganicae . Furthermore, as stipulated by Pellegrin (1938; see also Blache, 1964), the type locality is highly doubtful. In addition, the species is only known from its holotype and has not been found in the Chad Basin since then. As such, the holotype, which is in poor condition, is here identified as a mislabelled Capoeta species , possibly Capoeta trutta ( Heckel, 1843) (J. Freyhof, pers. comm., 2014). Indeed, although Pellegrin (1938: 373–374) did not explicitely mention a typical real cutting edge, and the specimen currently lacks one, this might have been fallen of, i.e. lost over time.
2. Two Southern African species have not been listed as valid species, i.e. Labeobarbus altidorsalis ( Boulenger, 1908) and Labeobarbus elephantis (Boulenger, 1907) . Both are here considered implicit synonyms (P.H. Skelton, pers. comm., 2015), respectively, of Labeobarbus codringtonii ( Boulenger, 1908) and Labeobarbus polylepis , following Skelton’s (1993, 2001) work (see annotated checklist 1).
3. Listing of all African species to be included into Labeobarbus is mainly hampered by the fact that during this study, at least one Barbus species previously identified as a small barb, i.e. Barbus urotaenia Boulenger, 1913 (see Table 1), was revealed to be a Labeobarbus species. Although described from small specimens, based on the presence of a free mental lobe and nine branched dorsal fin rays it is readily identifiable as a Labeobarbus species.
4. In addition, three nominal species, i.e. L. alluaudi , Labeobarbus microbarbis ( David & Poll, 1937) , and Labeobarbus microterolepis (Boulenger, 1902) , have been listed as hybrids or possible hybrids already. For L. alluaudi , the case has been convincingly documented by Banister (1972), and is followed here. For L. microbarbis , the case has been first suggest- ed by Banister (1973) and further documented by De Vos & Thys van den Audenaerde (1990), who confirmed Banister’s (1973) suggestion; however, for L. microterolepis , the case has only been suggest- ed by Banister (1973) and subsequently confirmed by Golubtsov, Dgebuadze & Mina (2002), but without providing new supporting evidence. The status of L. microterolepis as a possible hybrid thus certainly needs further attention. As hybrid nominal species are never formally synonymized, these nominal species already previously identified as hybrids or possible hybrids have been listed separately (see Table 1 and annotated checklist 2), as these names should not be used as valid names (see ICZN, 1999: article 23.8).
5. Varicorhinus beso was described from Lake Tana ( Ethiopia) by Rüppell in 1835 based on a single specimen, the holotype; however, as reported by Eschmeyer (2015) the SMF holotype cannot be found, a statement further confirmed by Mr T. Alperman (pers. comm., 2013), curator at the SMF, Frankfurt, Germany. As a result the holotype is here considered lost. The present situation is highly unsatisfactory for several reasons. Varicorhinus beso is the type species of the genus Varicorhinus and, although the genus Varicorhinus has recently been synonymized with Labeobarbus (see Berrebi et al., 2014), its status and delimitation as a valid genus has been (see Levin et al., 2013) and most probably will be further debated. Therefore, a neotype is needed (see ICZN, 1999: article 75), and is designated here, following the qualifying conditions provided in ICZN (1999: article 75.3). The NHM, London, UK, houses an unpublished, and as such unavailable, neotype (BMNH 1968.7.24.18; 156.8 mm SL), collected by Sandhurst in 1964, originating from Bahardar (Baherdar ± 11°37′N, 37°24′E), also on Lake Tsana (i.e. Tana, Ethiopia), and most probably selected by the late K.E. Banister (NHM); however, we have refrained from identifying this specimen as the neotype of V. beso . Instead, BMNH 1902.12.13.365 (290.6 mm SL), also originating from Bahardar, Lake Tsana (i.e. Tana, Ethiopia), and collected by E. Degen (1 June 1902), is here designated as the neotype of V. beso . The latter specimen has been illustrated by Boulenger (1907c: plate 33) in his Fishes of the Nile, and the illustration has been further reproduced in his monumental Catalogue of the Fresh-Water Fishes of Africa ( Boulenger, 1909: fig. 268). Boulenger (1907c: plate 33) illustrates a specimen of ± 293 mm SL (± 205 mm SL on the drawing, scale 7/10), which indeed can be identified as BMNH 1902.12.13.365 based on its size (SL) and exact disposition of the tubercles on the right-hand side of the head that perfectly match between the illustration and those on the NHM specimen. A detailed description of V. beso and a key to the species of the genus Varicorhinus as recognized at the time can be found in Boulenger (1907c, 1909). As such, the neotype exemplifies and corresponds with what has since been identified as V. beso . In addition, considering (1) the subsequent importance of the presence of a cornified, clear cutting edge as a diagnostic character for Varicorhinus , and (2) the general importance of the mouth phenotype and its polymorphism in these hexaploid Torini, the mouth of the neotype is in excellent condition and exemplifies a well-developed, cornified, clear cutting edge. This is especially important considering the fact that Rüppell (1835) himself did not mention the presence of a cornified, clear cutting edge (see Persistent generic problems: morphology, cytogenetic, and molecular approaches) as a diagnostic character for either the genus Varicorhinus or the species V. beso . Furthermore, the illustration of V. beso as provided by Rüppell (1835: plate 3; fig. 2) shows a fish without a clear cutting edge on the lower jaw, and in that respect looks more reminiscent of intermediate mouth phenotypes. Nevertheless, to stabilize the current nomenclature the neotype has been identified to match with what has been considered V. beso since Boulenger (1902f, 1906c; and more explicitly 1907c: plate 33).
6. As a result of the proposed ( Tsigenopoulos et al., 2010) and recently applied ( Berrebi et al., 2014) synonymy of Varicorhinus with Labeobarbus , and in concordance with other discoveries (see below), two valid species names become secondary junior homonyms, and are in need of a substitute name (see ICZN, 1999: article 60). A third case of secondary homonymy is also discussed, although a replacement name is not needed in this specific case. Finally, a fourth case of possible future secondary homonymy is also highlighted.
With regards to L. brevicauda , the right pharyngeal bone illustrated by Banister & Clarke (1980: fig. 5; 335 mm SL), and which according to the legend to the figure belongs to the holotype (ZMB 18175: 351 mm SL), does not in fact belong to this species. Indeed, the holotype has the left instead of the right pharyngeal bone dissected. This pharyngeal bone clearly has five teeth on row I and not four teeth, as in the illustrated pharyngeal bone. In addition, it has no molariform teeth at all. The drawing, however, perfectly matches the right pharyngeal bone, with four teeth on row I, of one of the syntypes of Labeobarbus latirostris Keilhack, 1908 (i.e. the whole specimen here designated as the lectotype, ZMB 18174, but not the head-only specimen, which here becomes the paralectotype, ZMB 34766; see below). The lectotype of L. latirostris also matches the size provided by Banister & Clarke (1980: 485; 335 mm SL) of the holotype of L. brevicauda . In addition, the barbels of the lectotype of L. latirostris are long, and therefore match the diagnosis given for Labeobarbus eurystomus by Banister & Clarke (1980: 489) rather than that for L. johnstonii [anterior barbels (Ab), mean 5.7% SL, and posterior barbels (Pb), mean 5.6% SL, in L. eurystomus , following Banister & Clarke (1980) = L. brevicauda , following Seegers, 1995 (see below), versus Ab 2.5% SL and Pb 3.5% SL in L. johnstonii ]. Banister & Clarke (1980) also referred to some typical differences in colour pattern, but we have been unable to confirm this as, at least in these specimens, the colour pattern is entirely faded. These discoveries strongly suggest that Banister & Clarke (1980) unfortunately seem to have switched parts of their synonymizations, as L. brevicauda was identified as a junior synonym of L. eurystomus (= L. brevicauda , following Seegers, 1995), although it has five non-molariform pharyngeal teeth on the first row, as in L. johnstonii , whereas L. latirostris was identified as a junior synonym of L. johnstonii , although it has four molariform teeth on the first row, unlike in the latter species. As a result, the nomenclatural consequences of these findings are twofold: (1) L. brevicauda becomes a junior synonym of L. johnstonii ; and (2) L. latirostris becomes the valid species name for what Banister & Clarke (1980) incorrectly referred to as L. eurystomus , and had been renamed by Seegers (1995) as L. brevicauda , following the invalid lectotype designation of Banister & Clarke (1980) for L. eurystomus (for more details, see annotated checklist 1 for L. johnstonii under the ‘Notes’ section), but unfortunately overseeing the additional errors of the latter authors (see above). The complete syntype of L. latirostris is here identified as the lectotype, as the second specimen (a head only), the paralectotype (now ZMB 34766), belongs to L. johnstonii (five teeth on row I and none of them molariform). As a result, a new replacement name (nomen novum; see ICZN, 1999: article 60.3) is needed for Varicorhinus latirostris Boulenger, 1910 , which also becomes a Labeobarbus and has no synonyms. Labeobarbus boulengeri is here proposed in acknowledgement of George Boulenger’s extensive work on Angolan large Barbus and Varicorhinus , both now Labeobarbus . For full details on the types see annotated checklist 1. The largest of the syntypes is here designated as the lectotype of the species (BMNH 1911.6.1.6: 136.6 mm SL), whereas the others become paralectotypes [ANSP 37905(1) (not seen), BMNH 1911.6.1.7–10(4), NMW 48865(1), and ZMB 18211(1)]. Indeed, following the recommendation of the ICZN (1999: recommendation 74B), the illustrated specimen should, by preference, be identified as the lectotype. The illustrated specimen in Boulenger (1916a: fig. 139) is about 95.0 mm SL. The scale provided is 2/5, which would give ∼ 237.5 mm SL. As the largest of the syntypes does not even come close to this size, we suspect the scale is in error and should most probably read 2/3 instead, as this would give an SL of approximately 142.5 mm, which is much closer to the 136.6 mm SL of the largest of the BMNH syntypes.
Varicorhinus mariae Holly, 1926 View in CoL has become L. mariae View in CoL . As a result, Barbus View in CoL (now Labeobarbus View in CoL ) mariae Holly, 1929 View in CoL described from the Kitui River in Kenya needs a substitute name (see ICZN, 1999: article 60). Banister (1973: 283) stated that although the original description of both L. matris ( Holly, 1928) View in CoL and L. mariae ( Holly, 1929) View in CoL were very similar, he refrained from putting them in synonymy without having seen the types; however, several authors ( Lévêque & Daget, 1984; Seegers, De Vos & Okeyo, 2003) have since considered B. matris View in CoL a synonym of B. mariae View in CoL , but with hesitation, without further justification, and without respecting the proper priority of names. The types of both species, previously considered lost, have been located in the NMW, and both nominal species are clearly distinguishable from each other, rejecting any claims regarding their possible synonymization. Amongst other character states, both nominal species differ from each other in the number of gill rakers [15 + 1 + 3 (=19) in B. matris View in CoL versus 9 + 1 + 2 (=12) or 10 + 1 + 2 (= 13), in B. mariae View in CoL ], the number of scales below the lateral line (3.5 versus 4.5), and the length of the unsegmented dorsal spine (18.8 versus 26.3–29.0% SL; E. Vreven, pers. observ., 2013). Therefore, both are here retained as valid species. Furthermore, Barbus View in CoL (now Labeobarbus View in CoL ) rhinoceros Copley, 1938 View in CoL has been identified as a junior synonym of B. mariae View in CoL by Banister (1973: 83). As a result, and according to ICZN (1999: article 60.2), it becomes the valid replacement name for the latter taxon. Contrary to Seegers et al. (2003), L. rhinoceros View in CoL is not considered a nomen nudum (see ICZN, 1999: glossary) as, according to ICZN (1999: article 13): (1) the name is accompanied by a brief description making reference to a ‘pronounced horn’ [see Copley (1938: 191); for an illustration see Banister (1973: fig. 68 for B. mariae View in CoL )], distinguishing it from all other East African Barbus View in CoL (now Labeobarbus View in CoL ); (2) although Copley (1938) seems to have had a single specimen (holotype) presented by Playford before him, a name bearing type designation only became mandatory for species descriptions after 1999 (see ICZN, 1999: article 72.3). No type(s) is/are known (see Lévêque & Daget, 1984; Eschmeyer, 2015); however, in the past, one and four additional specimens, all originating from the Athi River and presented to the NHM by Copley (BMNH 1936.12.22.35 and 1936.12.36–39, all currently in the same jar) have been labelled as the holotype and paratypes of B. rhinoceros View in CoL , respectively, but this has been subsequently amended. Therefore, and considering that (1) the specimens were deposited at the NHM in 1936, i.e. well before the actual description of the species in 1938, and (2) that Copley (1938) refers to a single specimen, the
type status of these specimens remains doubtful and in need of further research. Nevertheless, those are the specimens used by Banister (1973) for his detailed redescription of B. mariae View in CoL now B. rhinoceros View in CoL . For full details on the types of B. matris View in CoL and B. mariae View in CoL , also see annotated checklist 1. The suggested possible synonymy of both B. matris View in CoL and B. mariae View in CoL (now L. rhinoceros View in CoL ) with L. oxyrhynchus View in CoL (see Seegers et al., 2003: 32) is not followed, as no evidence was provided by the authors.
Labeobarbus macrolepis ( Pfeffer, 1889) View in CoL , a new combination first proposed by Skelton & Bills (2008), and later confirmed (see Banyankimbona et al., 2012a; present paper), is preoccupied by Labeobarbus macrolepis Heckel, 1838 View in CoL , currently a junior synonym of Tor putitora ( Hamilton, 1822) ( Kottelat, 2013) View in CoL . The junior secondary homonym L. macrolepis ( Pfeffer, 1889) View in CoL does not need a replacement name as: (1) ‘the junior species-group name has not been replaced’; and (2) ‘the relevant taxa are no longer considered congeneric’ ( ICZN, 1999: article 59.2).
Following Banister (1973), Varicorhinus stappersii Boulenger, 1917 View in CoL is to be considered a junior synonym of L. caudovittatus View in CoL (see annotated checklist 1); however, the syntypes of V. stappersii Boulenger, 1917 View in CoL all have a Varicorhinus mouth phenotype, whereas both syntypes of L. caudovittatus View in CoL have an intermediate mouth phenotype (see Table 1). Therefore, its current status as a junior synonym needs further attention. If, contrary to Banister’s (1973) opinion, further research reveals V. stappersii View in CoL to be a valid species instead, a replacement name will be needed for the former as it is preoccupied by Barbus View in CoL (now Labeobarbus View in CoL ) stappersii Boulenger, 1915 View in CoL . Being currently a junior synonym, however, it is not (yet) to be replaced, as the replacement name would be unavailable (see ICZN, 1999: articles 11.5 and 15.1).
7. The syntype series of L. sandersi , originally described as Varicorhinus sandersi by Boulenger (1912), is revealed to be polymorphic, including specimens with a real Varicorhinus cornified cutting edge, such as the one illustrated by Boulenger (1912: fig. 1, plate 19; see Table 1) in the original description (size of the drawn specimen: ± 315 mm SL), and specimens with an intermediate mouth phenotype, i.e. having a horny cover but lacking the real Varicorhinus cutting edge or Labeobarbus -like mental lobe (see notes to Table 1). Following ICZN (1999: recommendation 74B), the illustrated specimen (BMNH 1912.4.1.333; i.e. the largest of the syntypes, 316 mm SL), is here identified as the lectotype of the species. In addition, for reasons of nomenclatural stability, we have preferred to identify a specimen with a Varicorhinus mouth phenotype as the name-bearing type of L. sandersi rather than an intermediate one that might, in analogy to Banister’s (1972, 1976a) documented cases of hybridization, subsequently reveal to represent a hybrid mouth phenotype rather than a valid species.
8. The past recognition of Barbus as a ‘monstrous aggregation’ ( Myers, 1960: 213) has resulted in the synonymization of numerous genera. Following the recognition of Labeobarbus as a separate genus, several of these junior synonyms have to be reallocated to Labeobarbus instead of Barbus .
As such, in addition to the synonymization of Varicorhinus with Labeobarbus (see Berrebi et al., 2014), and taking into account all African Labeobarbus species identified in the present paper, at least three African junior synonyms are also to be listed under Labeobarbus : (1) Barbellion Whitley, 1931 ; (2) Barynotus Günther, 1868 (sensu Jordan, 1919), both with Barynotus lagensis Günther, 1868 ( Nigeria) as the type species; and (3) Lanceabarbus Fowler, 1936 (originally described as a subgenus of Barbus ), with Barbus tanensis Günther 1894 ( Kenya; a junior synonym of L. oxyrhynchus ; see annotat- ed checklist 1) as the type species. Further details on these junior synonyms are provided in the notes to Table 1 and in annotated checklist 1. As B. capensis is in fact not a Labeobarbus (see E. Vreven, E.R. Swartz & P.H. Skelton, unpubl. data) and the name L. seeberi should be used for this southern African species instead, Cheilobarbus Smith, 1841 (originally described as a subgenus of Barbus ) is not to be included as a junior synonym of Labeobarbus .
Although Pseudotor Karaman, 1971 , with B. fritschii ( Morocco) View in CoL as the type species, has been identified as a junior synonym of Carasobarbus View in CoL by Borkenhagen et al. (2011) and Borkenhagen & Krupp (2013), and the monospecific genus Pterocapoeta , with Pterocapoeta maroccana Günther, 1902 ( Morocco) View in CoL as the type species, has recently been revalidated by Borkenhagen et al. (2011), both are here includ- ed in Labeobarbus View in CoL s.l. pending further research. Furthermore, when considering Labeobarbus View in CoL s.l. several non-African genera are to be included as well. First, both Carasobarbus View in CoL , with Systomus luteus Heckel, 1843 ( Syria) View in CoL as the type species, and Kosswigobarbus , with Cyclocheilichthys kosswigi Ladiges, 1960 ( Turkey) View in CoL as the type species, are included here. Although both were referred to as subgenera in Tsigenopoulos et al. (2010), this subgeneric nomenclature was not adopted in their tree, despite both type species being included in their analyses. Furthermore, Berrebi et al. (2014) did not retain any subgeneric nomenclature either. Therefore, pending further research, both are included here in Labeobarbus View in CoL s.l. As stipulated elsewhere, we have refrained from following the revalidation of Carasobarbus View in CoL by Bănărescu (1997), as recently confirmed by Borkenhagen et al. (2011) and Borkenhagen & Krupp (2013), as this would render Labeobarbus View in CoL s.l. paraphyletic according to the results of Tsigenopoulos et al. (2010) and Berrebi et al. (2014). The same holds true for the monospecific genus Mesopotamichthys View in CoL , with Barbus sharpeyi Günther, 1874 View in CoL as the type type species, originally revalidated by Bănărescu (1997), as recently confirmed by Borkenhagen (2014), and the recently described genus Arabibarbus View in CoL , with Arabibarbus hadhrami Borkenhagen 2014 ( Yemen) View in CoL as the type species. Indeed, these three nominal genera are here all included in Labeobarbus View in CoL s.l. pending further research. Finally, although Tsigenopoulos et al. (2010) also included the (sub)genus Tor View in CoL , we have refrained from doing so as the type species itself, Cyprinus tor Hamilton, 1822 View in CoL , was not included in the analysis of Tsigenopoulos et al. (2010), nor in the compilation of Berrebi et al. (2014). Indeed, Tor View in CoL , including the type species T. tor View in CoL , has been inferred to be: (1) a valid genus, and to represent (2) the sister group to the Labeobarbus View in CoL clade, i.e. here referred to as Labeobarbus View in CoL s.l., by Yang et al. (2015: fig. 2) based on their mitochondrial DNA data set.
The present overview: what is to be learned?
Based on the tabulated overview (see Table 1), some revealing details with regard to the observed mouth phenotype diversity and its distribution are discussed: (1) valid African Labeobarbus spp. with one or several Varicorhinus or Capoeta genus or subgenus synonyms; (2) the generic history of L. wurtzi ; (3) other African species originally described based on one or several specimens with an intermediate mouth phenotype; (4) African Labeobarbus spp. with a prognathous mouth; (5) species originally described as Varicorhinus spp. but lacking the typical cornified cutting edge; (6) African Labeobarbus spp. , originally described as Varicorhinus spp. but with papillated lips instead of the typical cornified cutting edge; (7) details on the continental distribution of both the Labeobarbus and Varicorhinus mouth phenotypes.
1. Eight of the listed African Labeobarbus View in CoL s.l. species currently have a Varicorhinus junior synonym or originally a Capoeta View in CoL genus or subgenus one. These are: L. caudovittatus View in CoL (one Varicorhinus synonym), L. fritschii View in CoL (two Capoeta View in CoL synonyms = Varicorhinus ; E. Vreven, pers. observ., 2013), L. intermedius View in CoL (one Capoeta View in CoL synonym = Varicorhinus ; E. Vreven, pers. observ., 2013), L. johnstonii View in CoL (one Varicorhinus synonym), L. marequensis View in CoL (two Varicorhinus synonyms), L. oxyrhynchus View in CoL [two Capoeta View in CoL (subgenus) synonyms = Varicorhinus ; E. Vreven, pers. observ., 2013], L. trachypterus View in CoL (one Varicorhinus synonym), and L. tropidolepis View in CoL (one Varicorhinus synonym) (for more details, see notes to Table 1). Although Varicorhinus chapini Nichols & La Monte, 1950 View in CoL (currently a junior synonym of L. tropidolepis View in CoL , following Banister, 1973) was described as a Varicorhinus species , it lacks a cornified real cutting edge on the anterior edge of the lower jaw (for more details, see Table 1). This means that seven (i.e. 5.6%) valid African Labeobarbus species have, at least, one nominal junior synonym for which the lower jaw, somehow, bears a cornified real cutting edge as found in V. beso View in CoL (see Boulenger, 1907c: plate 33; Levin, 2012: fig. 2), now L. beso View in CoL . In addition, three additional Labeobarbus species , i.e. L. aeneus View in CoL , L. natalensis View in CoL , and Labeobarbus nthuwa Tweddle & Skelton, 2008 View in CoL have been reported to include Labeobarbus View in CoL as well as Varicorhinus mouth phenotype specimens (see Skelton, 2001 and Tweddle & Skelton, 2008), which according to Gaigher (1975) also holds true for L. polylepis View in CoL (four species, i.e. about 3%); however, the cutting edge is not always as well developed as in V. beso View in CoL , as it may clearly not cover the entire width of the anterior edge of the lower jaw and may also lack the ventral cover of the lower jaw (here referred to as the plastron). This is the case, for instance, in the two largest of the BMNH syntypes (BMNH 1911.6.1.39–41: 79.3 and 80.1 mm SL) of Labeobarbus rosae ( Boulenger, 1910) View in CoL . In addition, in these specimens the lateral sides of the lower jaw bear well-developed fleshy lips, illustrating additional intermediate mouth phenotype variation in need of further attention.
2. Labeobarbus wurtzi View in CoL , although originally described as a large Barbus species (see Pellegrin, 1908), was transferred to the genus Varicorhinus by Daget (1962: fig. 14), although not stated explicitly, based on the fact that, as stipulated in his description of the Guinean V. wurtzi View in CoL specimens he studied, the lower jaw has a horny cover with a striated surface forming a cutting edge (i.e. ‘bord trenchant’ see Daget, 1962: 72). Lévêque & Guégan (1990), however, based on both morphological as well as parasitological criteria (i.e. monogenea Dactylogyridae View in CoL fauna), transferred it back to the group of the large Barbus View in CoL , i.e. now Labeobarbus View in CoL . Indeed, although Lévêque & Guégan (1990) reported the wide mouth with the presence of a horny covering (‘étui corné’) on the lower lip, they did not mention the presence of the typical Varicorhinus cutting edge that Daget (1962), instead, had explicitly reported. Re-examination of the holotype of L. wurtzi View in CoL (MNHN 1908–0097; 112.0 mm SL) shows that, although damaged, the holotype still partially bears a horny cover on the lower jaw, but nevertheless lacks the typical Varicorhinus clear cutting edge and, in that respect, as well as by the presence of fleshy lips on the lateral sides of the lower jaw, has a typical intermediate mouth phenotype. The smallest of both the L. wurtzi View in CoL specimens from Kaba ( Guinea) examined by Daget (1962) (MNHN 1959–0153; two specimens of 127.5– 132.5 mm SL) has a real cornified Varicorhinus cutting edge on the lower jaw, however (see Table 1). In addition, verification of the MRAC and MNHN specimen holdings for L. wurtzi View in CoL showed that other specimens also have a typical Varicorhinus mouth phenotype [MRAC 1986-13-P-114 (124.2 mm SL), MNHN 1959–0153(2) (smallest of both: 127.5 mm SL), MNHN 1987–0689(3) (116.6–165.5 mm SL), MNHN 1988–1955(3) (both largest specimens: 172.5 and 175.6 mm SL), and MNHN 1991– 0519(1) (180.3 mm SL)]. These specimens illustrate that specimens with the typical Varicorhinus mouth phenotype are – although far less abundant compared with the numerous L. wurtzi View in CoL intermediate mouth morphotype specimens in the MNHN and MRAC collections – not absent from this part of Africa, and might well represent an undescribed Labeobarbus species with a Varicorhinus mouth phenotype.
3. The fact that, as for L. wurtzi View in CoL , a Labeobarbus species has originally been described on a single specimen or several specimens with one or several intermediate mouth phenotypes (see Table 1) is certainly not unique for the latter species. Indeed, this is, for instance, also the case for both L. micronema View in CoL syntypes (BMNH 1904.2.29.37–38) originating from the Kribi River Basin ( Cameroon), which both also lack the presence of a mental lobe, typical for the Labeobarbus View in CoL -like phenotype (see Boulenger, 1911a: fig. 57) (see Table 1). As for the L. wurtzi View in CoL holotype, however, both syntypes have well-developed lips on the lateral sides of the lower jaw (see Boulenger, 1911a: fig. 57). Furthermore, although both these specimens lack the typical real cornified cutting edge, they have a broad mouth like the typical broad mouth found in the Varicorhinus -like mouth phenotype, and in that respect clearly differ from the narrow mouth of the typical Labeobarbus View in CoL -like mouth phenotypes. This kind of mouth phenotype is also reminiscent of the mouth phenotype illustrated for the hybrid specimens identified by Banister (1972: fig. 15; 1976a: plate 2).
4. It is to be noted, however, that all species with a prognathous lower jaw also lack the presence of a mental lobe [see Labeobarbus aspius ( Boulenger, 1912) , Labeobarbus macroceps ( Fowler, 1936) , L. mariae , L. matris , and Labeobarbus progenys (Boulenger, 1903) , as riverine species; see also de Graaf et al. (2010: fig. 1) for the prognathous, i.e. piscivorous, Lake Tana Labeobarbus spp. : Labeobarbus acutirostris ( Bini, 1940) , Labeobarbus longissimus ( Nagelkerke & Sibbing, 1997) , Labeobarbus macrophthalmus ( Bini, 1940) , Labeobarbus megastoma ( Nagelkerke & Sibbing, 1997) , Labeobarbus truttiformis ( Nagelkerke & Sibbing, 1997) , and to a lesser extent Labeobarbus gorguari (see Nagelkerke & Sibbing, 1997, 2000)]. Two additional Lake Tana Labeobarbus species , i.e. Labeobarbus dainellii ( Bini, 1940) and Labeobarbus platydorsus ( Nagelkerke & Sibbing, 1997) , without a prognathous lower jaw, are also known to be piscivorous (see Nagelkerke & Sibbing, 1997; de Graaf et al., 2010) and at least some specimens of L. dainellii are known to have a small lobe. As such, the lack of a mental lobe does not seem to unequivocally point towards an intermediate, possibly hybrid, status of the nominal species concerned; however, a hybrid origin is not to be excluded, and is certainly worth further detailed investigation.
5. In six currently valid African Labeobarbus species (i.e. about 5%), all originally described within Varicorhinus , i.e. Varicorhinus altipinnis Banister & Poll, 1973 (Lufira River Basin, DRC), V. ansorgii (Quango River Basin, Angola), Varicorhinus fimbriatus Holly, 1926 (Sanaga River Basin, Cameroon), Varicorhinus lufupensis Banister & Bailey, 1979 (Lufupa River Basin, DRC), V. macrolepidotus (Kasai River system, DRC), and Varicorhinus wittei Banister & Poll, 1973 (Lufira River Basin, DRC), the name-bearing type(s) (i.e. the holotype or all examined syntypes) lack the typical cornified, real cutting edge on the lower jaw (see Table 1). In that respect, these name-bearing types also resemble some of the intermediates, i.e. hybrid phenotypes, as described and illustrated by Banister (1972: fig. 15; 1976a: plate 2). As a result, in analogy with the cases documented by Banister (1972, 1976a), these might well represent hybrid phenotypes instead of valid species.
6. Results also show that four currently valid Labeobarbus species (i.e. about 3%), namely Labeobarbus robertsi ( Banister, 1984) (Inkisi River Basin, DRC) (see Banister, 1984: fig. 9), Labeobarbus clarkeae ( Banister, 1984) (Quanza River Basin, Angola), Labeobarbus ensifer ( Boulenger, 1910) (Lucalla River Basin, Angola), and Labeobarbus varicostoma ( Boulenger, 1910) (Lucalla River Basin, Angola), all have papillae towards the anterior outer edge of the upper as well as the lower jaw (see Table 1). As all were originally described within the genus Varicorhinus , the existence of this additional, very distinct, mouth phenotype has largely been overlooked. Furthermore, this mouth phenotype seems, based on the current evidence, highly localized, as it has only been reported from the Quanza and Lucalla rivers in Angola and the Inkisi River, Lower Congo, in the DRC.
7. Based on the currently available data resulting from the compilation provided, Labeobarbus as well as Varicorhinus mouth phenotype(s) and/or species seem to be widespread in Africa, as they both apparently occur in each of the ten ichthyofaunal provinces (see Snoeks et al., 2011) recognized today (see Fig. 6 View Figure 6 and associated table). The spacial distribution of Varicorhinus mouth phenotype species seems to have been particularly obscured. Several reasons can be put forward for this: (1) numerous synonymizations of nominal Varicorhinus mouth phenotype(s) species with similar sympatric Labeobarbus species (see above under no. 1); (2) unnamed Varicorhinus mouth phenotypes, within the current context of the accepted high intraspecific mouth phenotype polymorphism within Labeobarbus (see above under no. 1); and (3) apparently entirely overlooked Varicorhinus mouth phenotypes in other cases (see above under no. 2, L. wurtzi ).
In addition, the overall continental distribution shows the Labeobarbus mouth phenotype(s) and/or species to be, apparently, more widespread compared with the Varicorhinus mouth phenotype and/or species. For instance, the Varicorhinus mouth phenotype is currently unknown from within the L. bynni region – i.e. large parts of the Nilo Sudanic ichthyofaunal province – including the Upper Senegal, Volta, and Niger, including the coastal rivers between both, as well as the Chad and the ‘Lower’ Nile Basin. The latter mouth phenotype is not entirely absent from the Nilo Sudanic ichthyofaunal province, however, and is expressed, among other taxa, in L. jubae , originally described as a Varicorhinus species , from the Juba River (see Banister, 1984; Levin et al., 2013; Table 1). In addition, the current state of knowledge should be taken cautiously. Indeed, as illustrated, the so-called established absence of the Varicorhinus mouth phenotype in the Upper Guinea ichthyofaunal province (sensu Lévêque, 1997: fig. 2.1.) is here refuted (see above under no. 2 for L. wurtzi ). Although rare, the re-examination of existing collections revealed it to be present in the area and confirms Daget’s (1962) statement in this respect (see above under no. 2, L. wurtzi ).
The present compilation has also enabled us to illustrate the taxonomic evolution of the African Labeobarbus s.l. species numbers since the original description of the genus in 1835 (see Fig. 7 View Figure 7 ). Several major trends can be observed. First, the highest number of described species per decade is between c. 1891 and c. 1940, which corresponds well with the period of intensive activity of G.A. Boulenger (1858–1937; NHM) and, later on, J. Pellegrin (1873–1944; MNHN) (see also Paugy, 2010: fig. 4; Skelton & Swartz, 2011). This activity curve is perfectly in tune with the expansion of exploration accompanying the colonial ‘scramble for Africa’ (see also Skelton & Swartz, 2011). Furthermore, although the cumulative number of valid species largely follows the trend of the cumulative number of nominal taxa up to c. 1960s, thereafter a period of intensive synonymization up to c. 1990 brought a strong decrease in the number of valid species. The conceptual shift in the interpretation of mouth phenotype variation also accounts for the peculiar history of the African valid Labeobarbus s.l. species numbers, which contrasts with the overall numbers of African fish species descriptions, and exemplifies the huge impact of the conceptualization of species on the practice of their recognition and delimitation. African fish species descriptions have been steadily increasing since the c. 1880s (see Paugy, 2010: fig. 5; Skelton & Swartz, 2011: fig. 1), but very few Labeobarbus s.l. species descriptions occurred after the active period of G.A. Boulenger and J. Pellegrin, c. 1930s. Also, revalidations have been rare except for the 1990s when numerous Lake Tana ( Ethiopia) endemic species were revalidated by Nagelkerke & Sibbing (1997) after the extensive over-synonymization with B. intermedius , now Labeobarbus , by Banister (1973).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Labeobarbus
| Vreven, Emmanuel J. W. M. N., Musschoot, Tobias, Snoeks, Jos & Schliewen, Ulrich K. 2016 |
Labeobarbus boulengeri
| Vreven, Musschoot, Snoeks & Schliewen 2016 |
Arabibarbus
| Borkenhagen 2014 |
Arabibarbus hadhrami
| Borkenhagen 2014 |
Tor putitora ( Hamilton, 1822 ) (
| Kottelat 2013 |
Labeobarbus nthuwa
| Tweddle & Skelton 2008 |
Pseudotor
| Karaman 1971 |
Carasobarbus
| Karaman 1971 |
Carasobarbus
| Karaman 1971 |
Kosswigobarbus
| Karaman 1971 |
Carasobarbus
| Karaman 1971 |
Mesopotamichthys
| Karaman 1971 |
Cyclocheilichthys kosswigi
| Ladiges 1960 |
Labeobarbus rhinoceros
| Copley 1938 |
rhinoceros
| Copley 1938 |
L. rhinoceros
| Copley 1938 |
B. rhinoceros
| Copley 1938 |
B. rhinoceros
| Copley 1938 |
L. rhinoceros
| Copley 1938 |
Labeobarbus nanningsi
| de Beaufort 1933 |
mariae
| Holly 1929 |
Sanagia velifera
| Holly 1926 |
Varicorhinus mariae
| Holly 1926 |
L. mariae
| Holly 1926 |
Varicorhinus stappersii
| Boulenger 1917 |
V. stappersii
| Boulenger 1917 |
V. stappersii
| Boulenger 1917 |
stappersii
| Boulenger 1915 |
Labeobarbus latirostris
| Keilhack 1908 |
Pterocapoeta
| Gunther 1902 |
Pterocapoeta maroccana Günther, 1902 ( Morocco )
| Gunther 1902 |
Barbus sharpeyi Günther, 1874
| Gunther 1874 |
Systomus luteus
| Heckel 1843 |
Labeobarbus macrolepis
| Heckel 1838 |
Labeobarbus nedgia Rüppell, 1835
| Ruppell 1835 |
Tor
| Gray 1834 |
Tor
| Gray 1834 |
Cyprinus tor
| Hamilton 1822 |
T. tor
| Hamilton 1822 |