Kincaidiana smithi Fend & Rodriguez, 2017
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.361 |
|
publication LSID |
lsid:zoobank.org:pub:F61CB5C7-B22E-4FAB-997A-BF99C7828C77 |
|
DOI |
https://doi.org/10.5281/zenodo.3851848 |
|
persistent identifier |
https://treatment.plazi.org/id/9485884B-2496-42B0-8488-5A1A89514F9C |
|
taxon LSID |
lsid:zoobank.org:act:9485884B-2496-42B0-8488-5A1A89514F9C |
|
treatment provided by |
Carolina |
|
scientific name |
Kincaidiana smithi Fend & Rodriguez |
| status |
sp. nov. |
Kincaidiana smithi Fend & Rodriguez View in CoL sp. nov.
urn:lsid:zoobank.org:act:9485884B-2496-42B0-8488-5A1A89514F9C
Etymology
Named for Prof. Jerry Smith, in recognition of his contributions to the ecology and management of Pacific coastal streams.
Material examined
Holotype
UNITED STATES OF AMERICA: a dissected worm, stained in hematoxylin and mounted in Canada balsam, California, Del Norte County, Smith River below forks, 8 Jun. 2003, S. Fend leg. ( USNM 1422281 About USNM ).
Paratypes
UNITED STATES OF AMERICA: 1 dissected, same data as for holotype ( USNM 1422282); 1 dissected, same data as for holotype ( CASIZ 220930); 1 sagittally sectioned, same locality as for holotype, 10 May 2009, S. Fend and P. Rodriguez leg. ( CASIZ 220928); 1 dissected, seep by South Fork Smith River, 10 May 2009, S. Fend and P. Rodriguez leg. ( CASIZ 220931); 1 dissected, same data as preceding ( MNCN 16.03/3102).
Additional material (all partially-mature)
UNITED STATES OF AMERICA: 1 sagittally sectioned, 2 dissected, 1 whole mount, 3 in alcohol, from type locality, 8 June 2003; 2 dissected, same locality as preceding, 10 May 2009.
Molecular data
COI and 16S sequences correspond to topotypic voucher CASIZ 220929 (details in Table 1 View Table 1 ).
Description
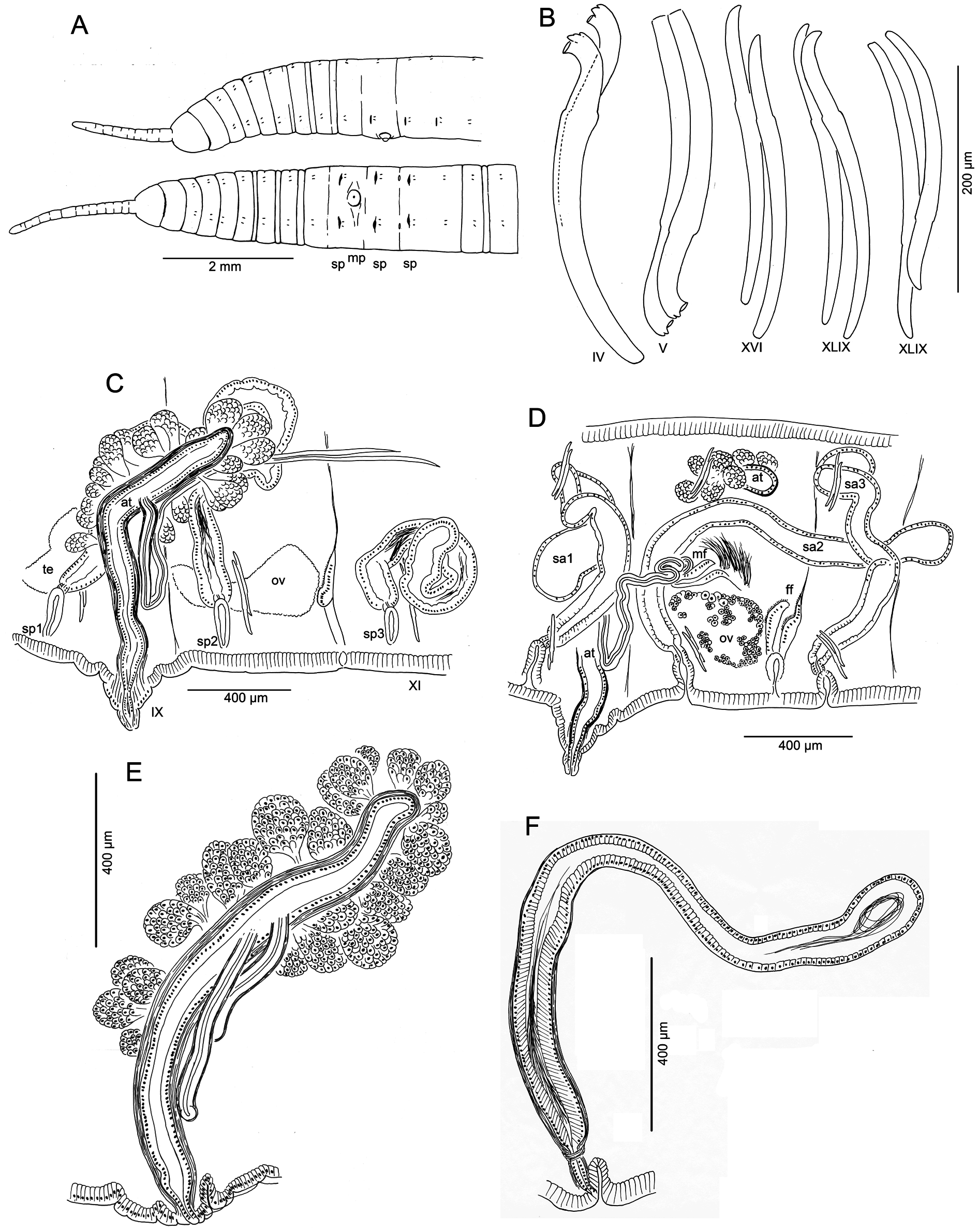
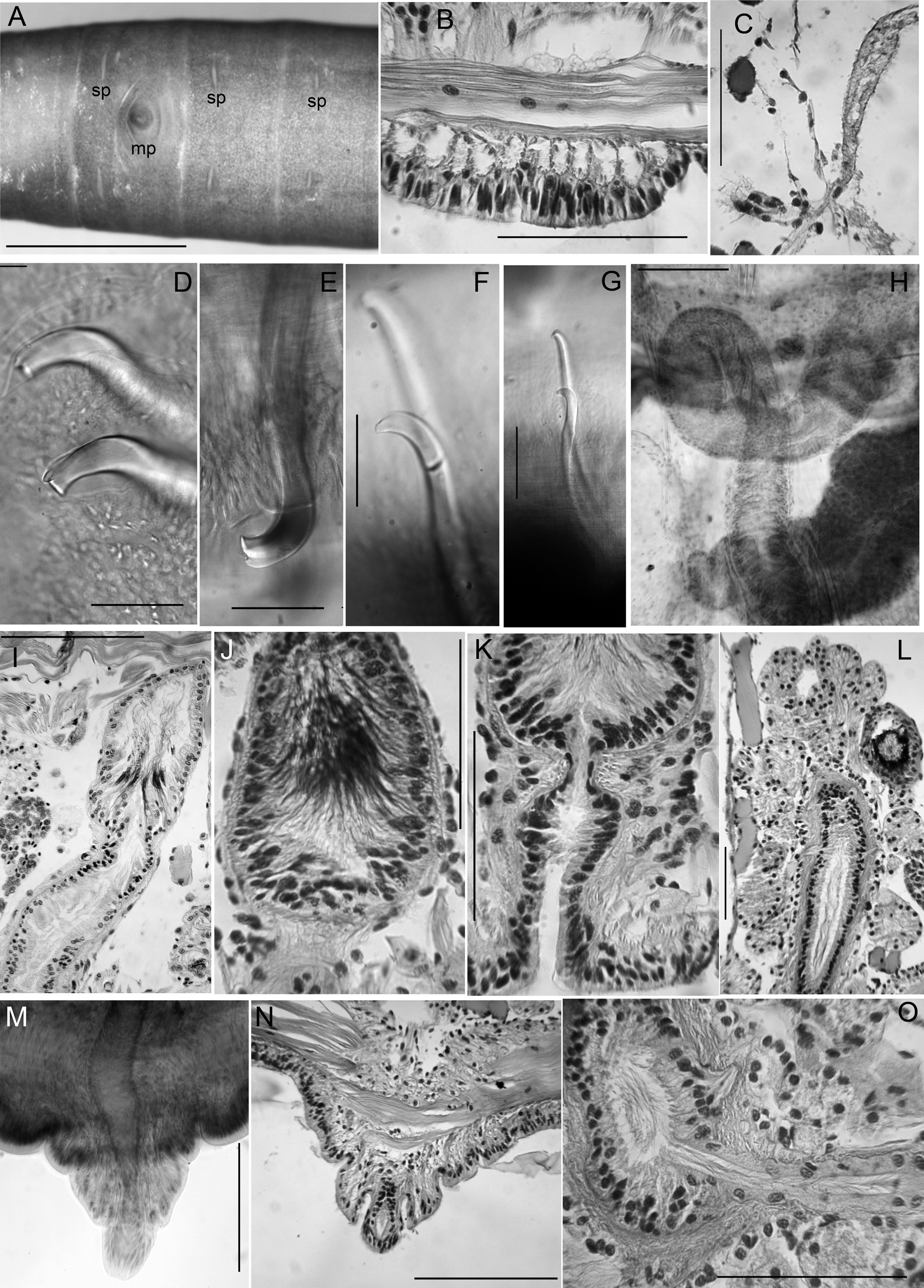
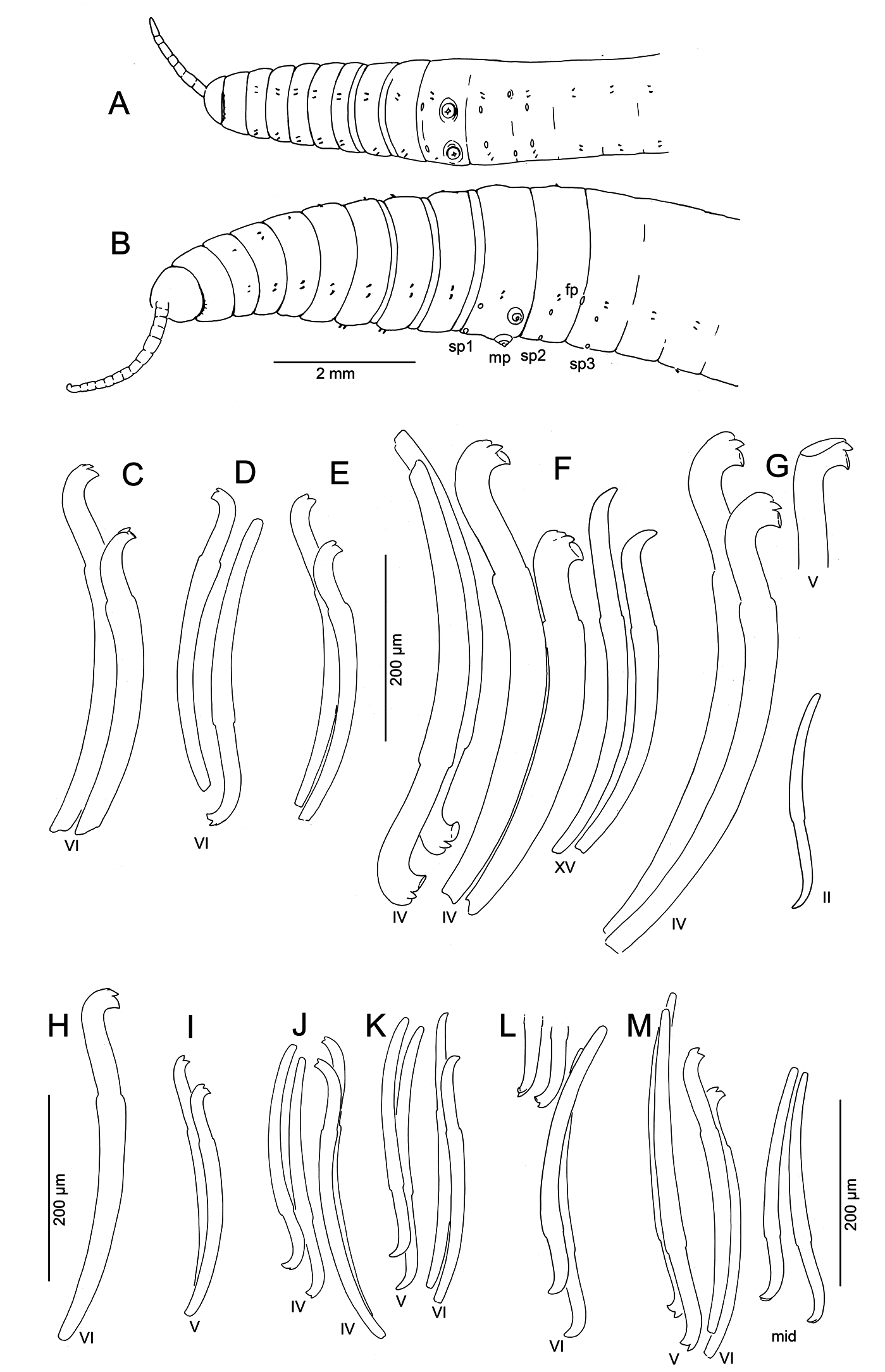
Medium-sized to large worms ( Table 2 View Table 2 ); prostomium short, length about ½ width; filiform proboscis 1–2 mm long, 0.1–0.16 mm diameter, not widened at base, externally ringed with multiple shallow constrictions ( Fig. 4A View Fig ). Body segmentation externally distinct in anterior segments, weak in clitellum and posteriorly; secondary annulation may appear as a narrow posterior ring in V–VII or VIII.
Chaetae two per bundle; those in II to (VII)VIII (IX) directed anteriad, others directed posteriad. Chaetae in II or III to VI or VIII appear bifid with large, flat ventral tooth and thinner dorsal tooth ( Fig. 5 View Fig D–E);
some chaetae with a thin dorsal keel above the upper tooth ( Fig. 4B View Fig ). Modified anterior chaetae strongly arcuate distal to the nodulus; shortest in II, gradually increasing in size from III to VI, maximum anterior chaeta length 270–470 μm. Within each pair, median (inner) chaeta has nodulus 0.3–0.4 the distance from the tip; lateral (outer) chaeta may be slightly thicker, with more distal nodulus, 0.25–0.3 distance from tip. Chaetae in IV–VII may be longer and thicker (width 15–22 μm) than more posterior chaetae (width 11–15 μm). Posterior to VI–VIII, chaetae usually simple-pointed ( Fig. 4B View Fig ), moderately sigmoid, and directed posteriad; dorsals about the same length as ventrals. Within each posterior pair, lateral chaeta has slightly more distal nodulus. Chaetae in segments posterior to about LXXX may be strongly arcuate; dorsal pairs may appear slightly bifid or with an upper keel ( Fig. 5F View Fig ).
Epidermis in anterior segments 25–32 µm thick; in clitellum 50–70 µm, thinner in posterior segments. Clitellum from about mid-VII to mid-XIII. In anterior segments, circular muscle layer of body wall arranged in a series of transverse bands with gaps between ( Fig. 5B View Fig ); this layer is 25–50 µm thick in pre-clitellar segments, gradually narrowing posteriorly down to 5–7 μm and appearing homogeneous. Longitudinal muscle layer of body wall 70–100 µm thick in anterior and middle segments. Pharynx from I–III or IV, with dorsal and lateral wall moderately thickened; transition to esophagus indistinct. Pharyngeal glands V–VII, with dorsolateral, median and ventrolateral lobes joining at posterior septa; lobes are joined between segments by thin extensions. No abrupt division between esophagus and intestine. Chloragogen cells cover gut beginning in VII. Brain in the peristomium, not deeply lobed.
Dorsal blood vessel separate from gut to about VIII, then closely appressed posteriorly. One pair of commissural blood vessels join dorsal blood vessel near posterior septum between II and about XX; these vessels lack a dense chloragogen layer; in pre-clitellar segments they are long and sinuous; those originating in II–VI join the ventral vessel(s) in the next segment; posteriorly, they are shorter, and join both dorsal and ventral vessels in the same segment. A pair of lateral, blind blood vessels, covered with chloragogen cells, joins the dorsal vessel in the anterior part of segments beginning in about XVI; at first, these are short and unbranched, but by XXV they reach the ventral part of the body, and have up to 10 long branches; by XL they may have more than 20 branches and fill much of the coelom. A second pair of blind, branched lateral blood vessels is located in the posterior part of each segment, posterior to about segment L. In more posterior segments (by about C), both pairs of lateral vessels have many short dorsal branches.
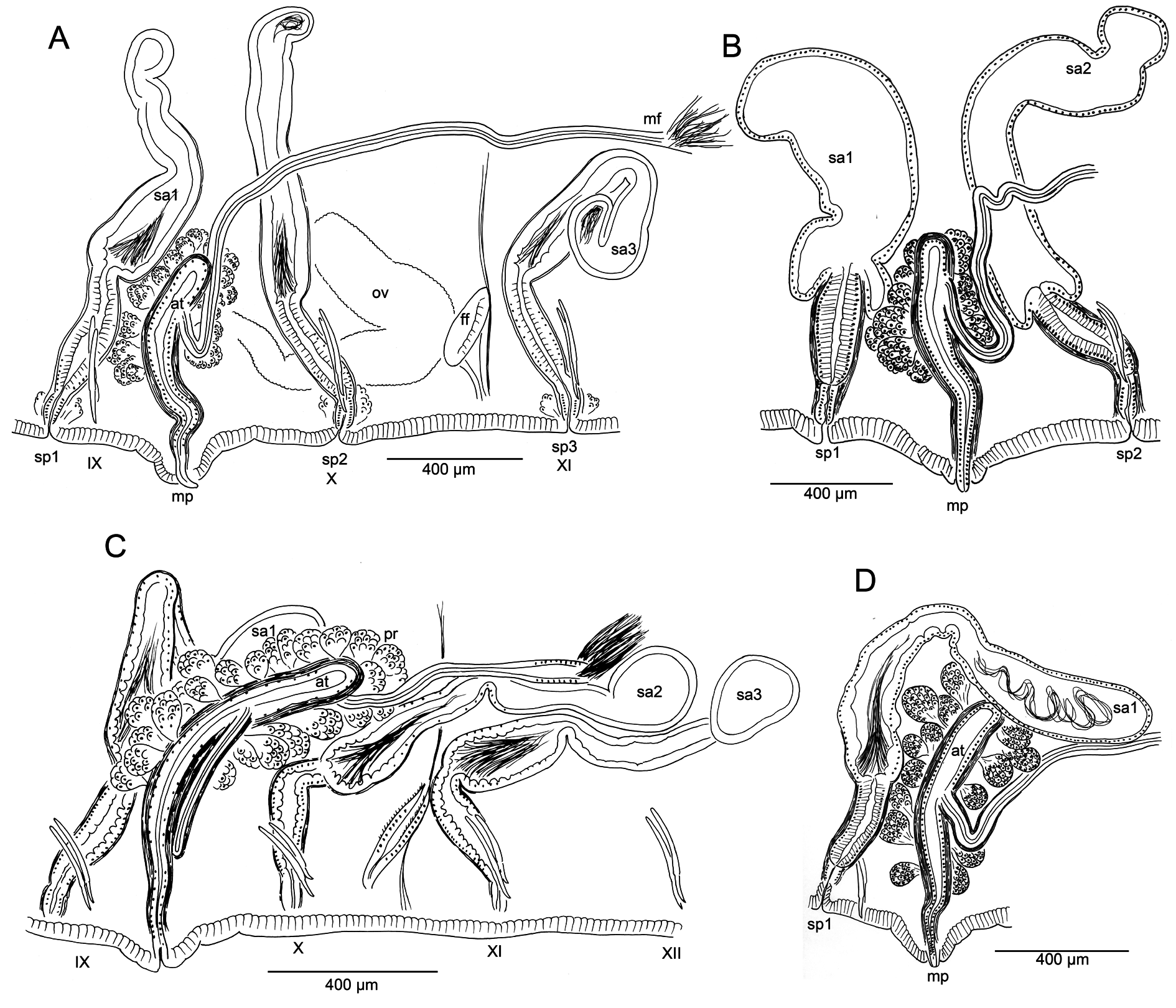
Nephridia usually paired on 11/12; occurring irregularly in posterior segments, absent from many segments. A small anteseptal funnel is followed by narrow, dorsally-directed, granular postseptal mass ( Fig. 5C View Fig ). Efferent duct forms a closely-appressed loop, extending to dorsal part of body cavity, forming a convoluted mass; duct ends in a narrow vesicle in front of ventral chaetae in the originating segment; nephridiopore inconspicuous.
Male pore single, median and posterior to ventral chaetae on IX; ectal tip of the atrial duct protrudes as a short penis. When everted, penis is subtended by a conical, tiered porophore ( Fig. 5 View Fig M–N), the entire structure up to about 200 μm high by 250–300 μm wide at base; porophore circular, usually consisting of two concentric epidermal folds. Spermathecae paired in IX, X, and XI; spermathecal pores on or very slightly median to ventral chaetal lines, all slightly anterior to respective chaetae ( Figs 4A View Fig , 5A View Fig ). One pair of testes in IX, reaching to mid-segment; one pair of ovaries in X, extending to back of segment or into XI; female funnels intersegmental in 10/11, up to 280 μm high. Sperm sacs extend posteriad to XVII–XXIV; no anterior sperm sacs; egg sacs with large eggs extend 1–2 segments behind sperm sacs.
Spermathecal ducts terminate within narrow vestibules, 100–130 μm deep; junction usually constricted by a muscular ring, forming a short sphincter ( Fig. 5K View Fig ). Spermathecae nearly tubular, about 1000– 2000 µm long, weakly divided into two sections, both of which may contain sperm ( Figs 4F View Fig , 5 View Fig H–J). Ectal third is duct-like, 100–130 μm wide, with uneven, columnar epithelium 25–50 μm thick, and distinct muscle layer 5–8 μm thick. Ental, ampullar section mostly tubular (about 75–85 μm wide), but may expand to 100–200 μm wide near ental end. Epithelium of ectal, duct-like section of spermatheca may be penetrated by heads of darkly-staining sperm cells which densely fill the lumen ( Fig. 5J View Fig ). Epithelium thinner (about 10 μm) in ental part of ampulla, and lumen wider, containing small amount of unordered, poorly staining sperm. Spermathecae in IX usually largest; those in XI smallest and may lack sperm. Spermathecae may extend posteriad through 1–2 segments ( Fig. 4D View Fig ).
Male pore without obvious glands, but surrounded by diffuse mass of muscle tissue. Single, prosoporous atrium is joined by both (posterior) vasa deferentia near the midpoint; they enter the atrial lumen directly at the junction ( Fig. 5O View Fig ). Atrium narrows ectally within the porophore; the remainder tubular, not divided into distinct regions, extending at least into mid-X ( Fig. 4 View Fig C–D); length 825–1620 μm, width 110–130 μm, including thick (12–25 µm) muscle layer. Atrial muscle layer thickest in ectal part, with fibers unevenly transverse to slightly diagonal, but not lined up (parallel) or in distinct layers; epithelium cuboidal, 10–15 μm thick. Ental ½–⅔ of the atrium covered by thick layer of multicellular prostate glands, 120–190 μm high; glands petiolate, broadly pyriform, with many cells ( Figs 4E View Fig , 5L View Fig ). Vasa deferentia 1200–1400 μm long, 34–40 µm wide; widest near the atrium (up to 40–48 μm), where they are covered by a thin muscle layer. Vasa extend posteriorly one or more segments, terminating in elongate, narrowly conical sperm funnels, up to 400 μm long.
Remarks
Both morphology and molecular results (see below) support K. smithi sp. nov. as a distinct species, closely related to K. hexatheca . Despite the morphological variability of K. hexatheca throughout its broad geographic range, K. smithi sp. nov. is distinctive in that all specimens from the type locality and a nearby site have a single, median male pore and atrium joined by both of the posterior vasa deferentia. The single, median atrium of K. smithi sp. nov. is unusual within the family, but this character appears in both species of Tatriella Hrabě, 1939 , as well as in some species of Eclipidrilus Eisen, 1881 (see Fend & Lenat 2012). As in K. smithi sp. nov., this arrangement does not usually represent a simple loss of one of the male ducts from the normal paired condition. In Eclipidrilus pacificus Fend, 2005 , for example, all four vasa deferentia join the median atrium ( Fend 2005: fig. 9B). Aside from rare and presumably teratological variation within populations, only Eclipidrilus ithys Brinkhurst, 1998 was described as having either one or two atria ( Brinkhurst 1998); but when only one is present, the entire male duct is missing from one side.
Compared with most specimens of K. hexatheca , the atrium in K. smithi sp. nov. is more elongate, usually entering the post-atrial segment; nevertheless, this occasionally occurs within the range of variation in populations of K. hexatheca ( Table 2 View Table 2 , Fig. 3C View Fig ). The spermathecal pores are clearly displaced from the ventral chaetae line towards the mid-body line in K. hexatheca , while in K. smithi sp. nov. the pores are on the chaetal line, anterior to the chaetal bundles ( Fig. 1 View Fig A–B vs 4A). The morphology of the spermathecae is similar to that of K. hexatheca ; however, they are typically more narrow-elongate, with faint distinction between ampullar and duct portions.
Other characters of K. smithi sp. nov. conform closely to those of its congener, K. hexatheca . Both have a large, rather cylindrical body, with a ringed proboscis. The chaetal morphology resembles that of typical K. hexatheca , and the distribution and orientation of modified anterior chaetae are also similar. Less conspicuous, but nevertheless unusual characters are also shared with K. hexatheca (see Fend 2009): the circular musculature of the body wall, the narrow, dorsally-directed nephridia, and the muscle layer extending along the ectal end of the vasa deferentia.
Habitat
The Smith River site is a large (average discharge> 100 m 3 /s), free-flowing stream with riffle-pool structure and gravel-boulder substrate. Specific conductance was 90 μS cm- 1 in April 2014 (62–150 μS cm- 1 in 1978–1981, NWIS 2016a). Kincaidiana smithi sp. nov. was only found in a backwater area with some silt deposition. The other collection site was a small roadcut seep, with slow current and some rooted aquatic vegetation. Guts were filled with undetermined organic matter and very fine mineral particles, with diatoms.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |