Juxtacribrilina annulata ( Fabricius, 1780 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5016.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:6E3BF843-16C1-4B91-AB72-D5C1D556384A |
|
persistent identifier |
https://treatment.plazi.org/id/03BCE061-A162-FFD8-B790-F9925CD3FD81 |
|
treatment provided by |
Plazi |
|
scientific name |
Juxtacribrilina annulata ( Fabricius, 1780 ) |
| status |
|
Juxtacribrilina annulata ( Fabricius, 1780) View in CoL
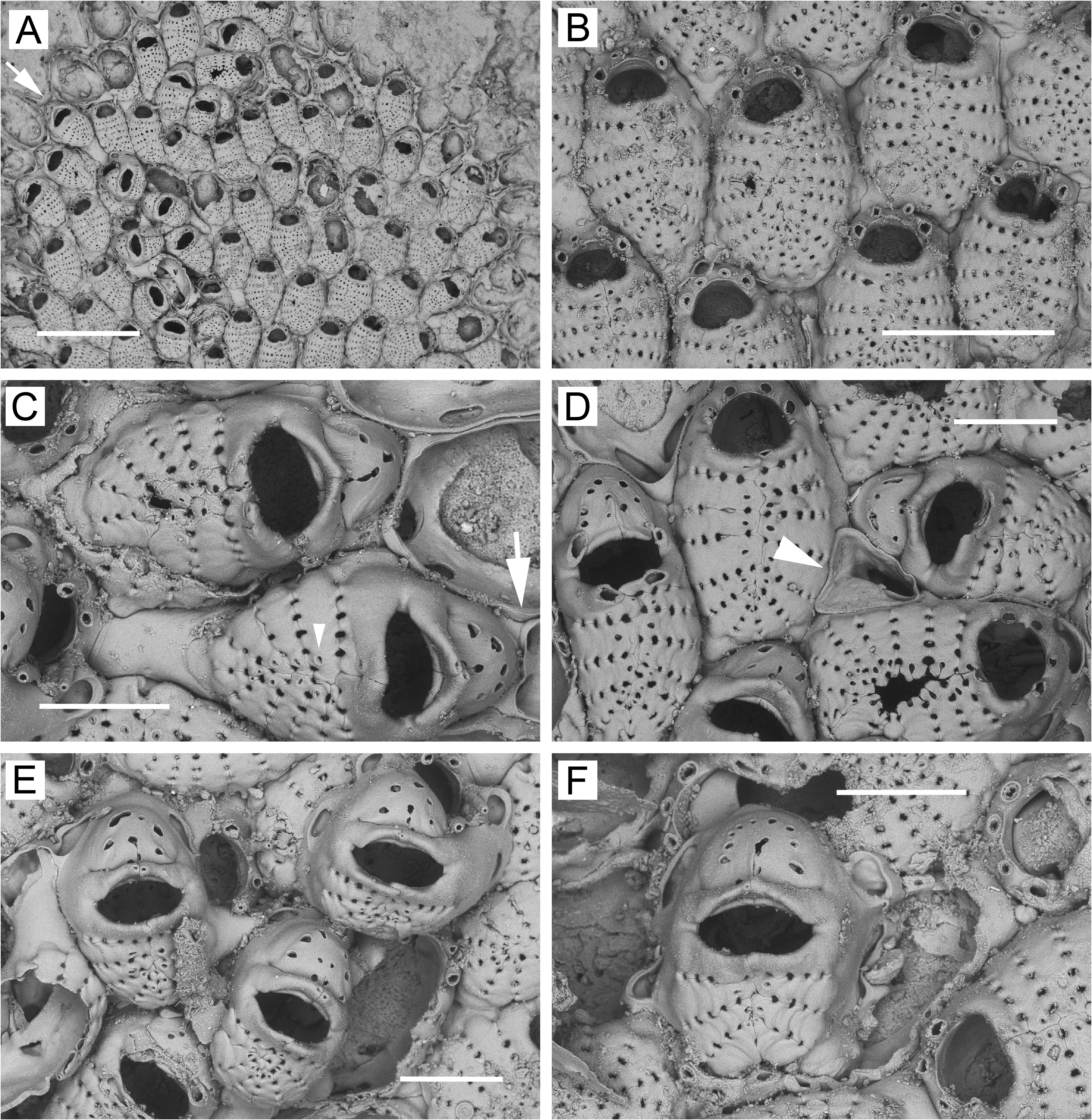
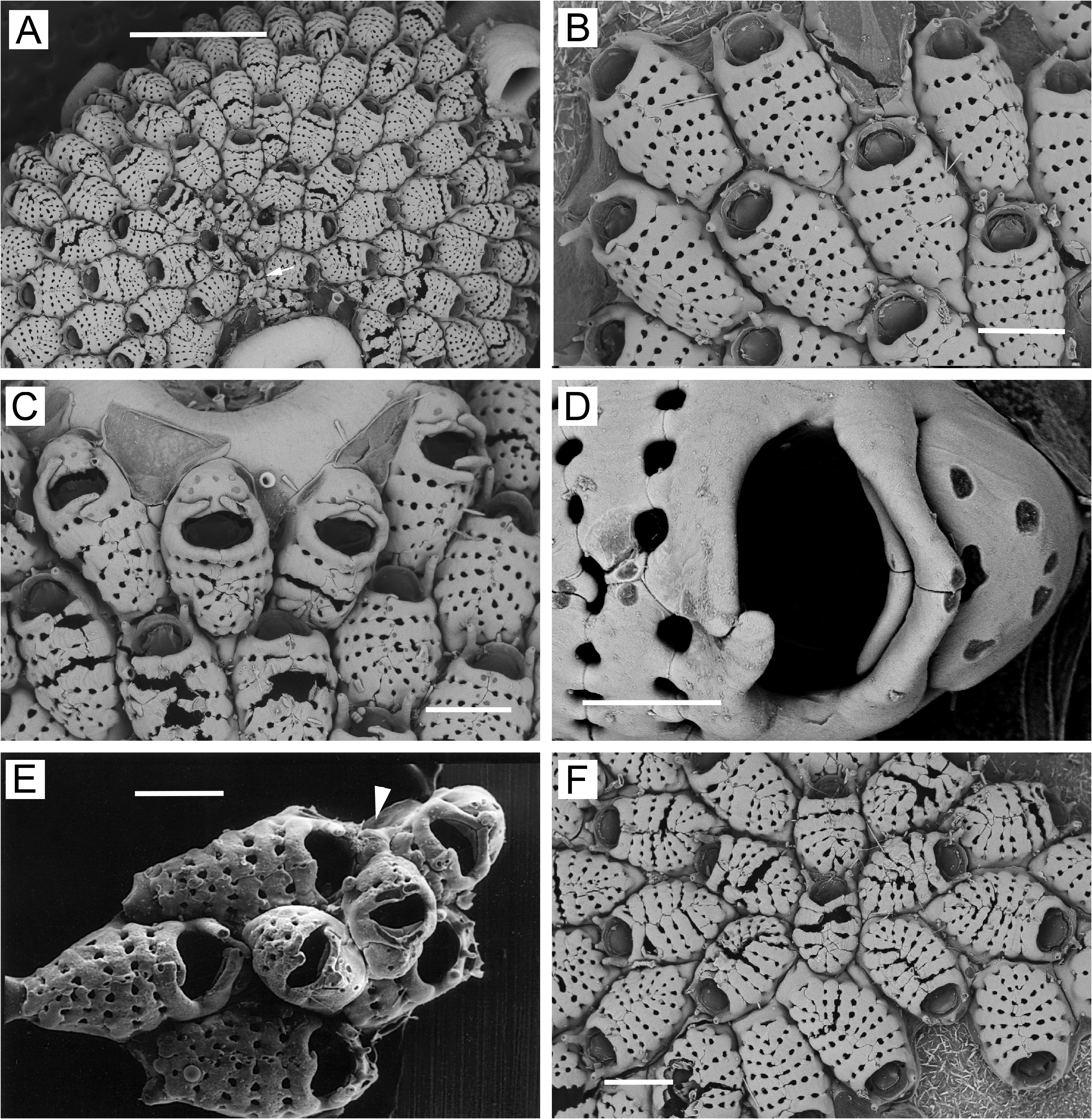
( Figs 2 View FIGURE 2 , 3 View FIGURE 3 ; Table 1)
Cellepora annulata Fabricius, 1780, p. 436 .
Cribrilina annulata: Osburn 1950, p. 177 View in CoL , pl. 28, fig. 7 (in part?, referring to Arctic material from Point Barrow, Alaska); Kluge 1975, p. 470, fig. 247; Bishop 1994, p. 232, figs 25–32; Hayward & Ryland 1998, p. 314, fig. 110; Ostrovsky 1998, figs 8–10, 13, etc.; Yang et al. 2018, figs 5–7.
not Cribrilina annulata: Dick & Ross 1988, p. 51 View in CoL , pl. 4C; Dick et al. 2005, p. 3718, fig. 7A, B; Grischenko et al. 2007, p. 1093, fig. 17.
Material examined. Specimen from NW Pacific region: ZIRAS 32 /50735, western Kamchatka Shelf, Sea of Okhotsk , KamchatNIRO Collection , RV Professor Probatov, Station 6 (57.46722°N, 156.43417°E), one colony on interior surface of bivalve shell fragment, collected by GoogleMaps T. B. Morozov, 16 June 2013, Sigsbee trawl, gravel bottom, 40 m depth . Other material examined : ZIRAS 33 /50736, White Sea, Kandalaksha Bay, Chupa Inlet , near Matrenin Island , close to the Belomorskaia Educational and Research Station , Saint Petersburg State University (66.30917°N, 33.62861°E), six colonies on algae, dried and mounted on SEM stubs, collected by O.N. Kotenko, 20 September 2020, dredge and GoogleMaps SCUBA diving, 5–10 m depth .
Measurements. See Table 1.
Description (Kamchatka specimen). Colonies encrusting, unilaminar ( Fig. 2A View FIGURE 2 ) except for reduced ovicellate zooids overgrowing zooids of basal layer ( Fig. 2E, F View FIGURE 2 ). Zooids oval or spindle-shaped in outline, delineated by deep groove, with little space between them ( Fig. 2B View FIGURE 2 ); gymnocyst negligible, sometimes exposed at proximal angles or on narrowing proximal part of zooid ( Fig. 2C, D View FIGURE 2 ); frontal pore chambers lacking. Frontal shield highly convex, comprising 13–17 costae (one abnormally large zooid with 20 treated as outlier, not included in measurements or average). Costae oval or flattened in cross section, with 8–10 intercostal lacunae in complete transverse series between them; in non-ovicellate zooids ( Fig. 2B View FIGURE 2 ), suboral pair of costae swollen, not wider than rest and often narrower, their tips sometimes slightly raised to form median projection. Each costa with single lumen pseudopore, at tip or somewhat proximal ( Fig. 2C View FIGURE 2 ) to tip. Non-ovicellate zooids with secondary orifice roughly semicircular, corners rounded; 4 (rarely, 3) blunt, articulated oral spines, lateral pair thicker than distal pair.
Ovicellate zooids present in basal layer ( Fig. 2C, D View FIGURE 2 ) toward periphery or at margin; approximately as large (including ooecium) as non-ovicellate zooids, with 9–12 costae, suboral pair somewhat swollen, as wide as or somewhat wider than adjacent pair, often bi-lobed at ends, with more-distal lobes sometimes turned distally to form slight median projection. Ooecium ( Fig. 2C–F View FIGURE 2 ) hood-like, terminal; kenozooidal, budded from maternal zooid, occasionally bearing 1 or 2 basal pore chambers distally or disto-laterally; with thickened, non-articulated pair of modified latero-oral spines, each with single lumen pore, extending across proximal part of ooecium, usually meeting at midline, forming smooth or chevron-shaped arc, but not obscuring proximal edge of ooecium. Ooecium with 5–10 pseudopores, 1–4 in center and rest around peripheral margin. Median suture usually evident in proximal ooecial margin, sometimes extending distally to center of ooecium. Secondary orifice somewhat lozenge-shaped, bilabiate due to thickened costae proximally and modified spines distally.
Ovicellate zooids present frontally ( Fig. 2E, F View FIGURE 2 ); similar to but somewhat shorter than ( Table 1) basal ovicellate zooids; ooecial complex ( Fig. 2E, F View FIGURE 2 ) as in basal ovicellate zooids; sometimes with 1‒2 basal pore chambers distolaterally ( Fig. 2E View FIGURE 2 ). Frontally positioned ovicellate zooids appear associated with reparative growth or disruptions to zooidal packing. No true dwarf zooids observed; one possible developing dwarf (arrowhead, Fig. 2D View FIGURE 2 ) evident as bud from basal pore chamber of zooid in basal layer.
Remarks. Powell's (1967) description of J. annulata , based primarily on material from Vancouver Island in the boreal eastern Pacific and Prince Edward Island in the boreal western Atlantic, is problematic, because these populations are distant from one another and are not conspecific, as is evident from Powell's illustrations (see also below). The most detailed description of Atlantic J. annulata is that of Bishop (1994), based primarily on material from the North Atlantic and the Atlantic sector of the Arctic, and including SEM images. Both of these studies assumed that J. annulata is a single species with a circumpolar, Arctic-boreal distribution and included at least some information from Pacific specimens.
Juxtacribrilina annulata was originally described from Greenland ( Fabricius 1780), with a minimal description and no illustrations. Although Bishop (1994) did examine two specimens from Greenland, his description was based largely on material from Hardanger Fjord, Norway. Assuming that Bishop had illustrated type material, Yang et al. (2018) inadvertently referred to Hardanger Fjord as the type locality, but to our knowledge material from Greenland has not been redescribed, nor has a type specimen been designated, and J. annulata remains poorly defined taxonomically. Nonetheless, specimens from the boreal North Atlantic and European Arctic share zooidal characters that likely apply to J. annulata and that differ in key aspects from those in boreal Pacific populations. For the purposes of our study, and until the identity of J. annulata can be fixed, we consider Bishop's (1994) description of J. annulata to be representative of that species.
The Kamchatka ( Fig. 2 View FIGURE 2 ), Hardanger Fjord ( Bishop 1994, figs 25–32), and White Sea ( Fig. 3 View FIGURE 3 ) populations of J. annulata show some differences among one another, such as in zooid size, the predominant number of oral spines, and the number of costae and costal lacunae ( Table 1). In addition, while no ancestrula was observed in the Kamchatka specimen, ancestrulae from the White Sea have only two oral spines ( Fig. 3F View FIGURE 3 ) (see also Nikulina 2002, fig. 18; Yagunova & Ostrovsky 2008, top photo and fig. 4), whereas the ancestrula from Hardanger Fjord illustrated by Bishop (1994, fig. 32) has four. These differences are minor compared to several major features shared by the three J. annulata populations, as follows.
1) Zooids in the basal layer have the marginal gymnocyst typically limited to a slight exposure along the proximal margin or in the proximal corners, and frontal pore chambers are lacking ( Figs 2B View FIGURE 2 , 3B View FIGURE 3 ; Bishop 1994, figs 25–30).
2) Ovicellate zooids occur in the basal layer, ranging (including the ooecium) from somewhat longer than to somewhat shorter than non-ovicellate zooids, but having fewer costae ( Figs 2A, C, D View FIGURE 2 , 3C View FIGURE 3 ; Bishop 1994, figs 25, 28, 29).
3) The ooecium is moderately well developed, hood-like, with a pair of modified latero-oral spines extending over the proximal surface and meeting at the midline, but leaving the ooecial proximal margin visible (e.g., Figs 2C View FIGURE 2 , 3D View FIGURE 3 ; Bishop 1994, fig. 28). The ooecium has a faint median suture showing on the proximal margin and sometimes extending as far as the center; there are 5–10 pseudopores (occasionally fewer), typically arranged with one to four near the center and the rest distributed around the distal curvature ( Figs 2F View FIGURE 2 , 3D View FIGURE 3 ; Bishop 1994, fig. 28), but sometimes appearing haphazardly arranged. Some ooecia in the basal layer possess one or two basal pore chambers distally or disto-laterally ( Fig. 3C View FIGURE 3 ; Bishop 1994, fig. 29) and occasionally bud a distal zooid from these chambers; Fig. 2C View FIGURE 2 shows one such instance.
4) Dwarf ovicellate zooids are relatively uncommon.We did not observe any fully dwarfed zooids in the Kamchatka specimen, nor were any evident in representative material that Bishop (1994) illustrated from Hardanger Fjord. Likewise, we found none among hundreds of small colonies on algae collected in 2020 from the White Sea, although they do occur in that population ( Fig. 3E View FIGURE 3 ; Ostrovsky 1998, fig. 13A; Nekliudova et al. 2019, fig. 5B– D), where their presence or absence is related to life history. In a study of J. annulata inhabiting algal substrates in the White Sea, Nekliudova et al. (2019) found that colonies produce no dwarf zooids in their first season of sexual maturity, but after overwintering produce frontal dwarf ovicellate zooids near the colony margin. In the White Sea population, frontal dwarfs are thus entirely absent from many colonies, as in our specimens from 2020.
By examining histological sections of White Sea specimens, Ostrovsky (1998) showed that frontally positioned dwarfs are not budded frontally, due to the lack of frontal pore chambers, but instead arise from basal pore chambers. The basally budded dwarfs are narrow as they extend interzooidally to the colony surface and then widen to form the frontal shield and ooecium ( Fig. 3E View FIGURE 3 , arrowhead; Ostrovsky 1998, fig. 14). Once a colony has formed, basal (ovicellate and non-ovicellate) zooids in the central region of the colony are unable to bud frontal dwarfs from basal pore chambers, because the latter are prevented from budding by the tight packing of the autozooids, and in most cases are already being used for interzooidal communication. This explains the general paucity of dwarfs in the interior of J. annulata colonies.
Occurrence. The presumed range of J. annulata is circum-Arctic ( Powell 1968; Kluge 1975), southward to Nantucket Island in the western Atlantic ( Osburn 1912), the Isle of Man in the eastern Atlantic ( Hayward & Ryland 1998), and western Kamchatka, Sea of Okhotsk (one record, this study) in the western Pacific. This nominal species, however, has not been adequately described from its type locality ( Greenland) or most of the rest of its range and potentially represents a complex of cryptic species.
| RV |
Collection of Leptospira Strains |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Juxtacribrilina annulata ( Fabricius, 1780 )
| Dick, Matthew H., Grischenko, Andrei V., Gordon, Dennis P. & Ostrovsky, Andrew N. 2021 |
Cribrilina annulata:
| Grischenko, A. V. & Dick, M. H. & Mawatari, S. F. 2007: 1093 |
| Dick, M. H. & Grischenko, A. V. & Mawatari, S. F. 2005: 3718 |
| Dick, M. H. & Ross, J. R. P. 1988: 51 |
Cribrilina annulata:
| Hayward, P. J. & Ryland, J. S. 1998: 314 |
| Bishop, J. D. D. 1994: 232 |
| Kluge, G. A. 1975: 470 |
| Osburn, R. C. 1950: 177 |
Cellepora annulata
| Fabricius, O. 1780: 436 |