Haenydra, Rey, 1886
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3607.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:97967754-52CD-4334-8714-F41C7A63B068 |
|
persistent identifier |
https://treatment.plazi.org/id/03EFC245-816B-FF96-FF1D-FF59A2F2F831 |
|
treatment provided by |
Felipe |
|
scientific name |
Haenydra |
| status |
|
" Haenydra " lineage overview
Morphology
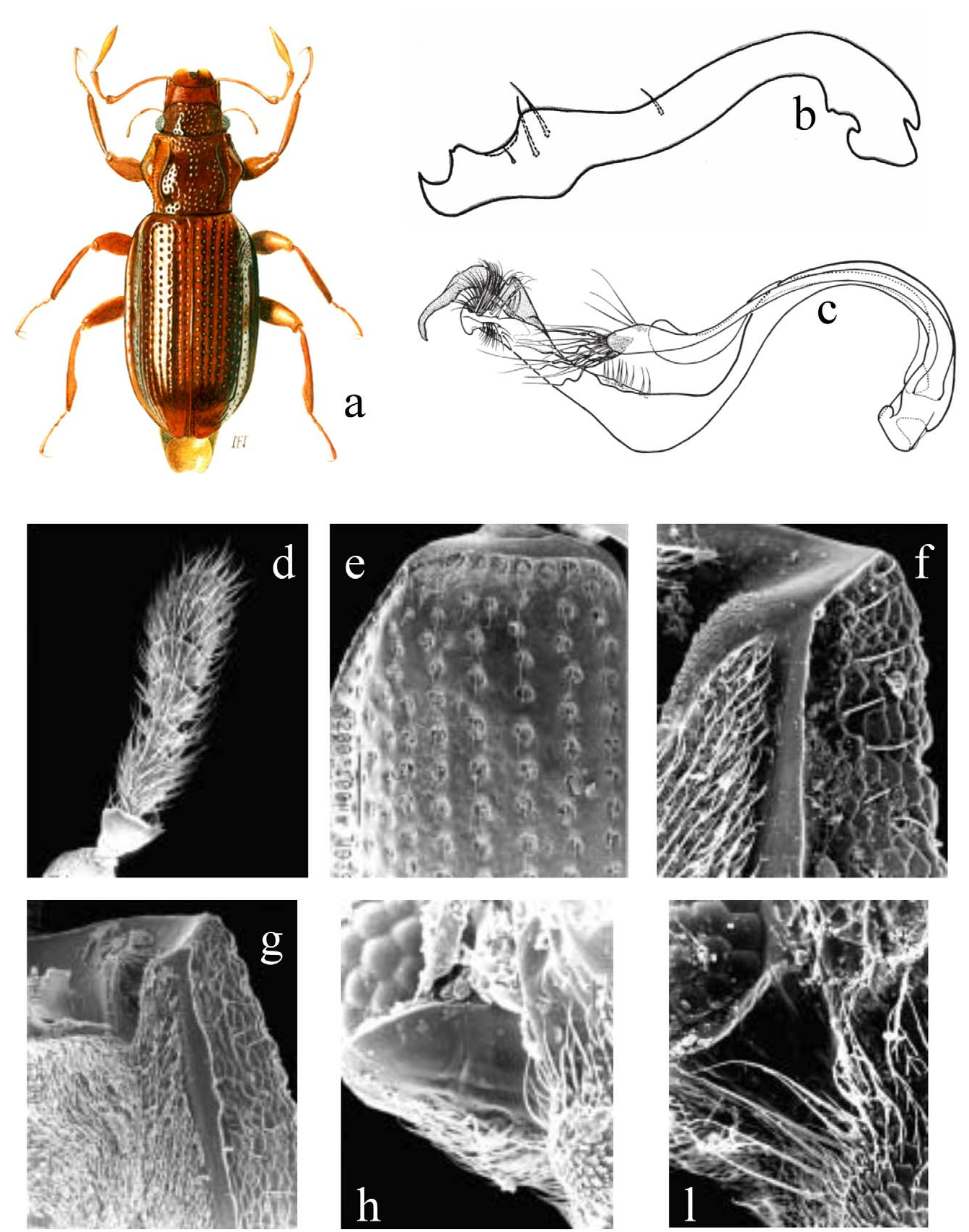
Hydraena spp. belonging to the " Haenydra " lineage are characterized by the relatively small body sizes (1.5 – 3.0 mm) and a narrow, flattened and elongate body shape ( Fig. 2a View FIGURES 2 ). They are usually dark brown to black, although a few species are completely reddish, orange or pale brown. Maxillary palps, legs and antennae (9 antennal segments as in all Hydraena s. l.; Fig. 2d View FIGURES 2 ) are often pale reddish brown, or, rarely, dark brown (e.g. H. vedrasi d’Orchymont ). Pronotum is usually cordiform or sub - hexagonal, with the pronotal disc notably convex (markedly more convex than most Hydraena s. str.), densely punctate near anterior and posterior margin, along midline, and the posterior foveae that are always deeply impressed ( Fig. 2a View FIGURES 2 ).
Elytra are elongate, often parallel - sided, with less than 10 parallel longitudinal rows of punctures between suture and humeri.
Females are characterized by seven uncovered ventrites, with segment VI smaller than V. In males there are six uncovered ventrites, with V smaller than VI. As in all Hydraenidae , the ovopositor is absent. Male secondary sexual characters are often strongly expressed in leg morphology (usually a row of more or less prominent teeth along the inner edge of the distal third on the mesotibiae, and a fringe of long setae along the mesal face of the distal half on the metatibiae, see Fig. 2a View FIGURES 2 ) and in the length of the maxillary palps, which are always longer than the antennae (as in all Hydraena s. l.). The shape of the female elytral apex and male genitalia are useful for species recognition.
" Haenydra " lineage species share the following diagnostic apomorphies (see d’Orchymont 1930a,b, Berthélemy 1986, Perkins 1997, and Jäch et al. 2000 for details and nomenclature of the mentioned characters):
absence of parameres in the male genitalia ( Figs. 2b,c View FIGURES 2 );
apex of antennal cupule rounded ( Fig. 2d View FIGURES 2 );
elytral striae straight and reduced in number, typically <10 per elytra ( Fig. 2e View FIGURES 2 );
apex of median subapical projection of gonocoxite distinctly raised;
hypomeral setae significantly reduced in size and number, often absent ( Fig. 2f View FIGURES 2 );
hypomeral secretion delivery (hsd) surface flat, completely smooth, and equally wide on both sides of hsd - sulcus;
closure of hypomeral antennal pocket exclusively formed by mesal, and never lateral, hypomera ( Fig. 2g View FIGURES 2 );
conspicuous convex spiculate area located posterior to genal antennal groove in postocular area ( Figs. 2h,l View FIGURES 2 ).
Ecology
Most " Haenydra " species usually live in a layer of nearly stationary water, where they adhere to the submerged stones mainly within the fastest regions of cold, clean and fastflowing perennial streams, mostly between 500 and 1500 m a.s.l (Figs. 3,4). Thus, the best way to collect these beetles is using an entomologic dredge ( Figs. 5a,b View FIGURES 5 ), and sifting stones both in the fastest central longitudinal sections and on the shallower and less turbulent areas on the sides of streams. Few species (e.g. Hydraena schuleri Ganglbauer ) are found shaking moss near falls within streams, whereas other ones (e.g. H. pangaei Jäch , H. carniolica Pretner and H. tarvisina Ferro ) live in small springs and streams characterized by reduced amounts of water flow ( Fig. 6 View FIGURE 6 ), and even in hyporheic waters. In summer, these springs are often depleted to only a humid soil with a few drops of water remaining, thus, during the dry season, these species may be collected using a tea strainer.
Some " Haenydra " species live almost exclusively in streams with siliceous substrates (e.g. H. dentipes Germar , H. lapidicola Kiesenwetter , and allies), while other species prefer carbonatic substrates (e.g. H. solarii Pretner , and allies). However, most of species are adapted to live in both these lithological substrates.
The majority of known adults of the " Haenydra " lineage feed upon diatomaceous algae. Larval stages of Hydraenidae have been poorly studied and the ecology of " Haenydra " larvae remains unknown, however they likely colonize the wet microhabitats surrounding the river bed. Adults of " Haenydra " may be collected from January to December, with the greatest concentrations of specimens typically found from July to September. The higher numbers during these months is likely due to a combination of factors including: reduction of water during the dry season, summer population peaks, and other abiotic and biotic conditions.
" Haenydra " beetles respire by means of trapping air via a plastron, which allows them to survive perenially submerged in running waters (Audisio unpublished data; Perkins 1997). The details of their “breathing - behavior” were comprehensively described by Perkins (1997).
Biogeography
" Haenydra " species are frequently endemic to extremely narrow areas (several species are known only from the type locality or from a single mountain massif), and are thus important from both conservation and zoogeographic standpoints. Nevertheless, this lineage also includes some species with wide geographic ranges, such as Hydraena gracilis Germar which is distributed across much of Europe, eastwards to the Urals, including Great Britain. Likewise, H. truncata Rey ranges from the northern Iberian Peninsula to Ukraine ( Jäch 2004; Ribera et al. 2011).
The most likely mode of speciation for these beetles (“speciation within refugia”) was recently hypothesized by Ribera et al. (2011), in a work dealing with the biogeography of the Iberian " Haenydra ", based on DNA sequence data. In this model, species likely originated in the areas in which they are currently found, due to the fragmentation (frequently during Plio-Pleistocene glacial cycles) of a single and more widely distributed species ( Fig. 7 View FIGURE 7 ).
The currently known 89 species of " Haenydra " are distributed in the northern Mediterranean region from Iberia to Iran ( Hansen 1998; Jäch 2004). One record of Hydraena exasperata (an Iberian endemic) is known from northern Morocco ( d’Orchymont 1935), but, as suggested by the same author, it must be considered a labelling mistake or a specimen carried over from the previous collecting activity in southern Spain ( Ribera et al. 2011).
The widespread species H. gracilis is the only " Haenydra " member currently known from the northern European Islands (i.e. Great Britain and Ireland). Ribera et al. (2011) suggested that this species represents a recent colonization of northern Islands during a period of lower sea level (likely during early Holocene last glacial maximum) that allowed land connections between these Islands and mainland Europe.
Three sibling species, H. evanescens , H. tyrrhena , and H. rosannae , are the only known species from Corsica ( H. evanescens ) and Sardinia ( H. tyrrhena and H. rosannae ) ( Binaghi 1961; Audisio et al. 2009). Basing on molecular data, the colonization of these two Mediterranean Islands from southwestern Europe likely occurred at the end or just after the Messinian salinity crisis (ca. 4.0–4.5 MY ago; Trizzino et al. 2011b), however other possible scenarios have been discussed for the colonization of Corsica and Sardinia by " Haenydra " species ( Audisio et al. 2009; Ribera et al. 2011).
Aims of the paper
The paper has two main objectives:
i) review taxonomy, morphology, biogeography and ecology of all species belonging to the " Haenydra " lineage;
ii) provide a faunistic dataset including all (or nearly all) published and unpublished known localities for each species, and a graphical output for each species complex (including the detailed geographic occurrence of each species).
During the preparation of the present revisional work, four new species were described ( H. rosannae Audisio, Trizzino & De Biase 2009 , from Sardinia; H. diazi Trizzino, Jäch & Ribera 2011 , from Spanish and French Pyrenees; H. fosterorum Trizzino, Jäch & Ribera 2011 , from N Spain, and H. gracilidelphis Trizzino, Valladares, Garrido & Audisio 2012 from Iberian Peninsula. These species were described in previous contributions and not in the present revision because of the involvement of other authors who contributed to species discovery and description but not to the present revision.
All the known 89 species are herein redescribed, providing also information about diagnostic characters, taxonomic and phylogenetic relationships, ecology and biogeography.
Finally, dichotomous keys were provided for identification of males of all the " Haenydra " species. Keys to identification of females are excluded, due to the low level of interspecific morphological differentiation in " Haenydra " females, mostly involving only the shape of elytral apex, elytral sides, and pronotum, which are otherwise easier to appreciate by direct cross-comparison with males of each identified and locally associated species.
State of the art of the " Haenydra " lineage
Checklist
" Haenydra " lineage species checklist (with synonyms), in alphabetical order; numbers between parentheses refer to the ordinal number of each species, used below both in species cards and keys to identification. Subspecies were not considered in the whole manuscript.
1. (50) akbesiana ( Audisio, De Biase & Jäch 1993)
2. (43) alpicola Pretner 1931 septentrionalis Pretner 1931 saga auctorum (partim)
3. (6) altamirensis Díaz Pazos & Garrido Gonzalez 1993
4. (34) anatolica Janssens 1963
5. (4) aroensis ( Ferro 1991)
6. (45) belgica d’Orchymont 1930
7. (79) bensae Ganglbauer 1901
8. (20) berthelemyana Jäch, Díaz & Dia 2006
9. (81) bicuspidata Ganglbauer 1901
10. (82) bitruncata d’Orchymont 1934
11. (63) bononiensis Chiesa 1959
12. (71) bosnica Apfelbeck 1909
ganglbaueri Fiori 1904
13. (2) carniolica Pretner 1970
14. (53) cata d’Orchymont 1943
15. (83) catalonica Fresneda, Aguilera & Hernando 1994
16. (12) caucasica Kuwert 1888
17. (55) christinae Audisio, De Biase & Jäch 1996
excisa ssp. esclusa d’Orchymont 1931 (partim)
18. (36) crepidoptera Jäch 1992
19. (65) czernohorskyi J. Müller 1911
20. (44) dalmatina Ganglbauer 1901
21. (60) decolor Sainte Claire Deville 1903
22. (84) dentipes Germar 1842
23. (67) devillei Ganglbauer 1901
24. (68) devincta d’Orchymont 1940
25. (39) diazi Trizzino, Jäch & Ribera 2011
saga auctorum (partim)
26. (87) discreta Ganglbauer 1904
prolongata Fiori 1904?
27. (33) elisabethae Jäch 1992
28. (38) emarginata Rey 1885
29. (29) epeirosi Ferro 1985
30. (59) evanescens Rey 1884
31. (56) exasperata d’Orchymont 1935
32. (51) excisa Kiesenwetter 1849
erosa Kiesenwetter 1849
33. (22) fontiscarsavii (Jäch 1988)
34. (40) fosterorum Trizzino, Jäch & Ribera 2011
saga auctorum (partim)
35. (76) gaditana Lagar & Fresneda 1990
36. (31) gracilidelphis Trizzino, Valladares, Garrido & Audisio 2012
gracilis auctorum (partim)
37. (30) gracilis Germar 1824
crassipes Mulsant 1844
38. (32) graciloides (Jäch 1988)
39. (11) gynaephila Jäch 1997
40. (85) heterogyna Bedel 1898 1
bidentata Ganglbauer 1901
binaghii Jäch 1989
doderoi Ganglbauer 1901
portai Fiori 1904
41. (41) hispanica Ganglbauer 1901
42. (19) hosseinieorum Bilton & Jäch 1998
43. (72) hungarica Rey 1884
44. (5) iberica d’Orchymont 1936
45. (52) integra Pretner 1931
ponticola Janssens 1968
46. (28) jaechiana ( Audisio & De Biase 1990)
47. (16) khnzoriani Janssens 1968
48. (25) krasnodarensis Jäch & Díaz 2006
49. (69) lapidicola Kiesenwetter 1849
50. (37) larissae Jäch & Díaz 2000
51. (18) lazica Janssens 1963
52. (73) leonhardi Breit 1916
53. (8) lusitana Berthélemy 1977
54. (7) madronensis Castro, Garcia & Ferreras 2000
55. (10) magnessa Jäch 1997
56. (75) manfredjaechi Delgado & Soler 1991
57. (77) monstruosipes Ferro 1986
58. (66) muelleri Pretner 1931
59. (35) nike Jäch 1995
60. (17) nilguenae (Jäch 1988)
61. (62) occitana (Audisio & De Biase 1995)
solarii auctorum (partim)
62. (21) orthosia Jäch, Díaz & Dia 2006
63. (48) pangaei Jäch 1992
64. (49) pelops Jäch 1995
65. (54) phallica d’Orchymont 1930
excisa ssp. esclusa d’Orchymont 1931 (partim)
66. (23) planata Kiesenwetter 1849
67. (15) plastica d’Orchymont 1943
68. (86) plumipes Rey 1886
procera Ganglbauer 1901
69. (80) polita Kiesenwetter 1849
70. (88) producta Mulsant & Rey 1852
1. See "Taxonomic remarks" in the species’ card
71. (26) prokini Jäch & Díaz 2006
72. (58) rosannae Audisio, Trizzino & De Biase 2009
73. (42) saga d’Orchymont 1930
74. (47) samnitica Fiori 1904
75. (70) sanfilippoi (Audisio & De Biase 1995)
76. (89) sappho Janssens 1965
77. (1) schuleri Ganglbauer 1901
78. (9) scitula d’Orchymont 1943
79. (13) septemlacuum Jäch 1992
80. (14) sinope Jäch 1992
81. (61) solarii Pretner 1930
82. (24) solodovnikovi Jäch & Díaz 2006
83. (3) subintegra Ganglbauer 1901
84. (46) tarvisina ( Ferro 1991)
85. (74) tatii Sáinz Cantero & Alba Tercedor 1989
86. (64) truncata Rey 1885
falzonii Pretner 1930
87. (57) tyrrhena Binaghi 1961
88. (27) vedrasi d’Orchymont 1931
89. (78) zezerensis Díaz Pazos & Bilton 1995
Systematics and phylogeny
Two centuries of taxonomic research on Hydraenidae , especially on " Haenydra " species, have provided a broad knowledge base for these beetles. In particular, species belonging to this lineage may be divided into several complexes ( Table 1), based on the shape of the male genitalia, and, to a lesser extent, on external morphology. Trizzino et al. (2011b) carried out a molecular phylogeny of the whole lineage, using both nuclear and mitochondrial markers. Molecular analyses strongly supported the monophyly and derived phylogenetic position of the “ Haenydra ” lineage, and four major monophyletic lineages were recovered: Hydraena gracilis , H. caucasica , H. dentipes , and the H. iberica lineages. The clades including H. iberica and allies and H. caucasica and allies were retrieved within an isolated position in " Haenydra ". The other two major lineages ( H. dentipes and H. gracilis lineages) included several monophyletic clades and different sub-clades, with the latter often corresponding to previously identified species complexes (see Table 1).
Using a calibration of 0.01 substitutions/site/MY ( Ribera et al. 2011), the origin of the “ Haenydra ” lineage was estimated to be at ca. 8.5 MY (Tortonian), with a wide confidence interval. The two main lineages ( Hydraena gracilis and H. dentipes lineages) split ca. 7.6–7.8 MY ago, with the major clades likely originating between ca. 4 and ca. 6 MY ago (Messinian), while the majority of terminal clades and clusters of allopatric sibling species were estimated to be less than 3.0 MY old (Plio-Pleistocene origin, as a consequence of Glacial Cycles).
Species with widespread distributions displayed two different patterns. Relatively deep divergences were observed within some of the widely distributed species, such as H. polita (ca. 0.8 MY between specimens from the Pyrenees and southern Germany), or H. truncata (0.9 MY between specimens from northern Spain and Central Italy); in contrast, H. gracilis , the most widespread “ Haenydra ”, exhibited minimal molecular differences among specimens throughout its geographic range ( Latvia, Britain, Bulgaria; Italy, Greece; see also Ribera et al. 2011), strongly suggesting a recent, likely late Pleistocenic and post-Wurmian, expansion of the species’ range.
...... continued on the next page
H. bensae Ganglbauer 1901 —Italian and French Maritimes Alps
H. bicuspidata Ganglbauer 1901 —Central E France (E Central Massif)
H. bitruncata d’Orchymont 1934 —French/Spanish Pyrenees and Central Spain
H. catalonica Fresneda, Aguilera & Hernando 1994 — NE Spain (Catalonia)
H. polita Kiesenwetter 1849 —Central W Europe
H. tatii complex ( Delgado & Soler 1991)
H. gaditana Lagar & Fresneda 1990 —SW Spain (Cadiz province)
H. manfredjaechi Delgado & Soler 1991 —S Spain (Jaen province)
H. tatii Sáinz Cantero & Alba - Tercedor 1989—S Spain (Sierra Nevada Massif)
...... continued on the page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Haenydra
| Trizzino, Marco, Carnevali, Lucilla, Felici, Stefano De & Audisio, Paolo 2013 |
Haenydra
| Rey 1886 |