Eucalyptus odorata, Behr, Behr
|
publication ID |
https://doi.org/10.1071/SB21029 |
|
DOI |
https://doi.org/10.5281/zenodo.10974607 |
|
persistent identifier |
https://treatment.plazi.org/id/5261160A-FF8B-FFD2-BB67-401FFEC0B6A2 |
|
treatment provided by |
Felipe |
|
scientific name |
Eucalyptus odorata |
| status |
|
Relationships within the E. odorata View in CoL complex
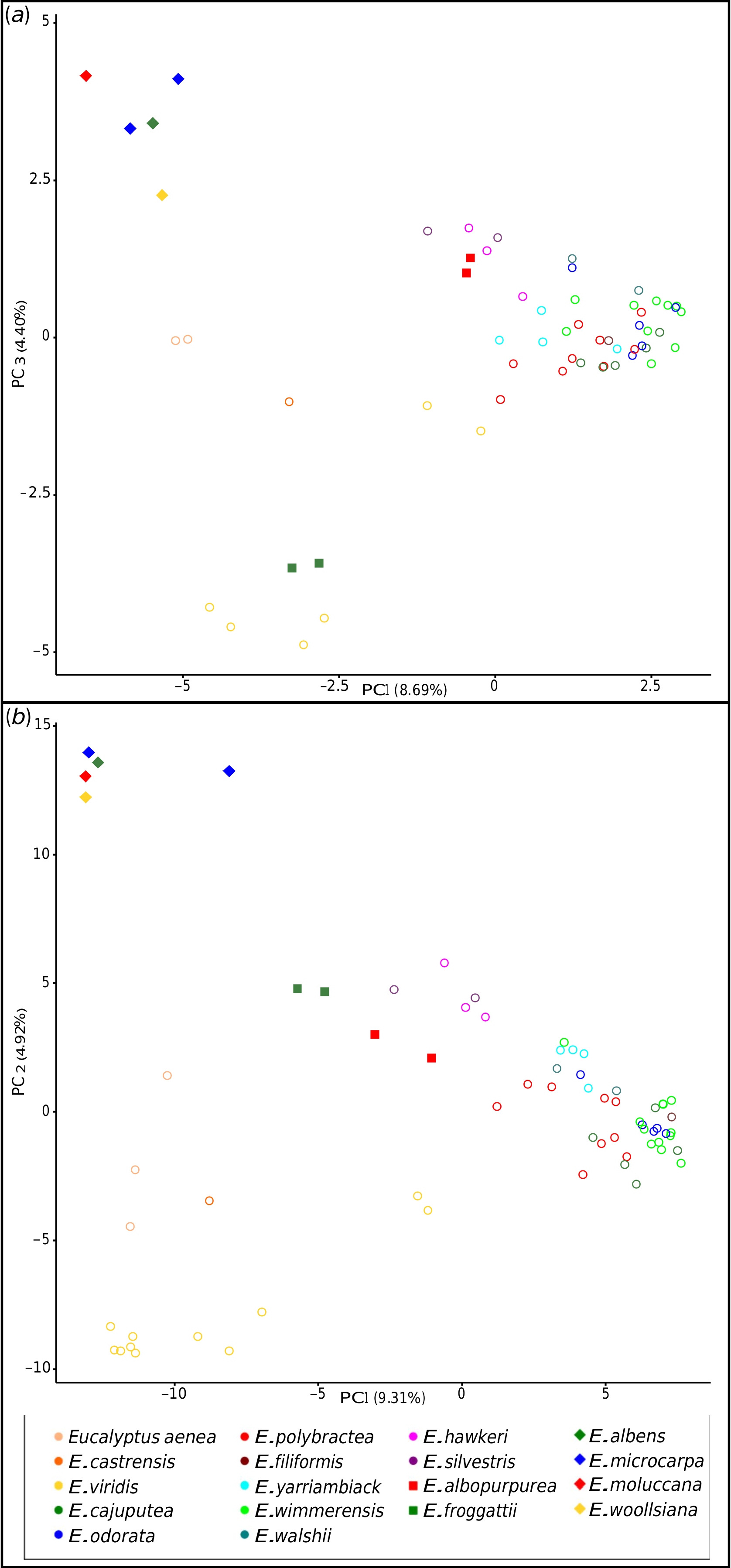
Relationships within the E. odorata complex are largely unresolved in our phylogeny, with very little bootstrap support in both the MP and ML analyses, and no species with multiple populations sampled being resolved as monophyletic. However, our PCA ( Fig. 3 View Fig ) and hybridisation tests ( Tables 4 View Table 4 , 5 View Table 5 ) have shed some light on the possible patterns of relatedness and introgression that explain this lack of resolution. There is strong support for the idea that E. viridis and the two segregate species from the Hunter Valley, E. aenea and E. castrensis , form a clade sister to the rest of the complex if northern ( Queensland) populations currently regarded as E. viridis are excluded. Although E. viridis co-occurs with E. polybractea at multiple locations and the two may hybridise on occasion (but no evidence of this was seen in this study), the former is the most morphologically distinct species in the complex, given its linear juvenile leaves and its narrow, green adult leaves, and, at most sites, the two are easily recognisable and morphologically distinct. The two Hunter Valley segregates of E. viridis , E. aenea and E. castrensis , form a sister lineage to E. viridis in our phylogeny. However, the PCA ( Fig. 3 View Fig ) and hybridisation tests ( Tables 4 View Table 4 , 5 View Table 5 ) give weight to the hypothesis that these populations have experienced introgression from a grey-box species, which may account for their morphological distinctness, although given the results of our ABBA-BABA tests ( Table 4 View Table 4 ), E. albens appears the probable parent rather than E. moluccana as was hypothesised by Nicolle (2019). As the NewHybrid analysis assigns these samples to E. viridis rather than to a hybrid generation, this finding of introgression from a grey box is likely to be the result of historic introgression. The genetically distinct sample of E. aenea ( PSF 90 J) was recognised as differing from smooth-barked typical E. aenea in the field because of its stocking of rough bark that reached ~ 3 m up the trunks. Although genetically distinct from the other E. aenea samples in our dataset, this is not due to more substantial genetic input from a grey box as hypothesised. Although grey boxes are the most closely related species to occur in the vicinity of E. aenea , ironbark species of E. section Adnataria ( E. crebra F.Muell. , and E. fibrosa F.Muell. ) dominate the site and may be the culprit for this genetic distinctness, although our dataset does not allow us to test this. As our E. castrensis sample is more referable to the broader application of the name per Hill and Stanberg (2002) that may represent E. aenea , and we have not sampled material from the putative E. aenea × E. microcarpa – E. moluccana hybrids, which match the type material of the species ( Nicolle 2019), we cannot say anything further regarding the distinction of E. castrensis from E. aenea .
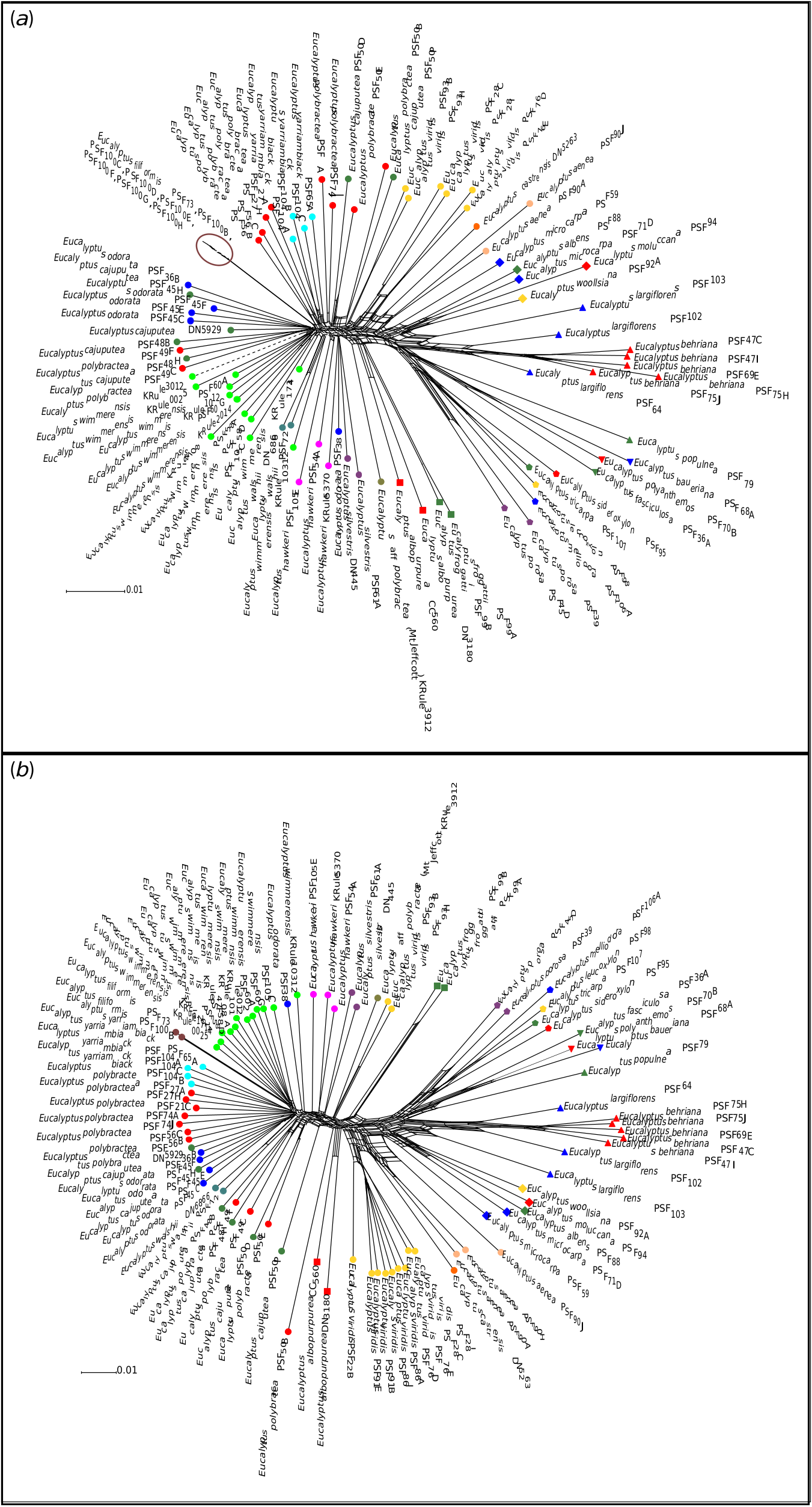
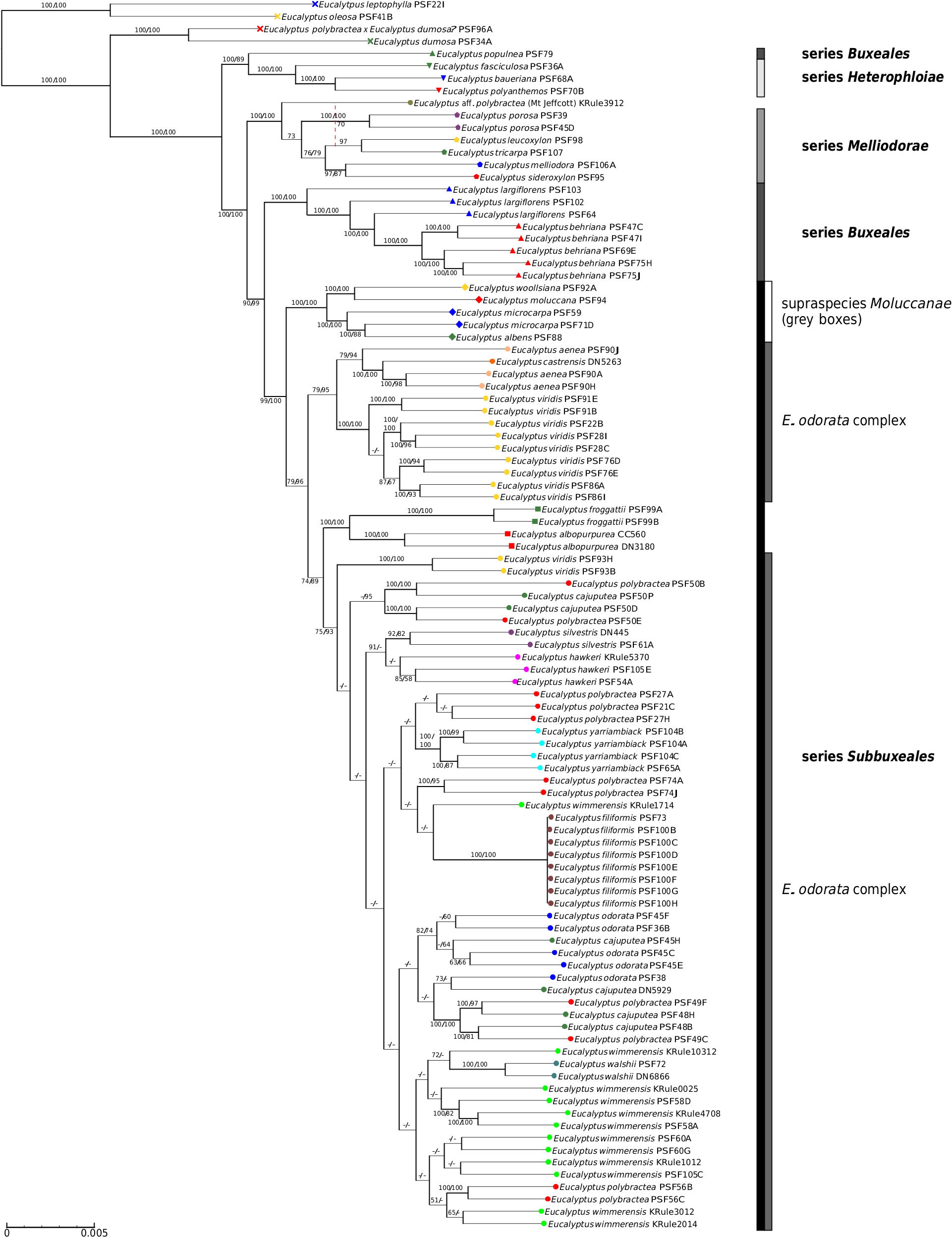
Previous authors have noted that Queensland populations in the vicinity of Inglewood, where the two northern E. viridis samples included in this study were collected, and Durikai State Forest have broader leaves than typical for E. viridis , with Blakely (1934) classifying these populations as E. viridis var. latiuscula Blakely. Chippendale (1988) suggested that these populations may be hybrids of E. viridis and the grey-box E. woollsiana , whereas Brooker et al. (2015) believed that these populations show greater morphological similarities to E. wimmerensis , which they regard as a subspecies of E. viridis , than to the typical form of E. viridis found in the Victorian goldfields and scattered populations in NSW. In the more resolved reduced sampling phylogeny ( Fig. 5 View Fig ), the northern samples are placed as the next clade to diverge in the complex after the main E. viridis clade, with the samples of E. polybractea from the most northerly population diverging next, although the node between these clades is supported only in the ML analysis. The ABBA-BABA tests also do not support introgression from the co-occurring grey-box E. woollsiana into these samples compared with E. viridis and E. polybractea from West Wyalong ( Table 4 View Table 4 ), as was previously hypothesised ( Chippendale 1988). Along with the placement of these samples in the PCA analyses ( Fig. 3 View Fig ), this raises the question of whether rather than an E. viridis × E. woolsiana hybrid, they may be E. viridis × E. polybractea hybrids, although given the lack of E. polybractea populations nearby (the nearest known population at West Wyalong being over 820 km away), they would have to be phantom hybrids, which have been observed in eucalypts previously, albeit without such a large geographic distance to the phantom parent ( Kirkpatrick et al. 1973; Hopper and Wardell-Johnson 2004). However, our ABBA-BABA tests showed no support for this hypothesis ( Table 4 View Table 4 ), with the northern E. viridis samples showing a similar number of shared alleles with typical E. viridis as our samples of E. polybractea from the most northerly population of this species at West Wyalong. This leaves the simplest explanation as the most probable, in that these northern populations previously regarded as E. viridis var. latiuscula represent a currently unrecognised distinct entity that is sister to the core E. odorata complex rather than being closely related to typical E. viridis , which fits with the observation of morphological similarities to E. wimmerensis of seedlings from populations previously ascribed to this variety at Inglewood and Durakai in Queensland by Brooker et al. (2015).
Relationships between the few supported clades and the majority of samples in the core E. odorata complex are unsupported, despite the topology returned broadly reflecting geography. Only the taxa known from a single site, namely E. yarriambiack and E. filiformis , are supported as monophyletic ( Fig. 5 View Fig ), and because we have sampled all known wild individuals of E. filiformis , we can say with confidence on the basis of the lack of genetic differences between them, that the species represents a single clonal colony. Previously it has been noted that E. filiformis does not appear to not readily reproduce ( Rule 2004), although specimens grown at the Royal Botanic Gardens Victoria from seed show differences in adult morphology from the wild individuals ( Rule 2018). We have sampled one of these cultivated individuals (PSF73) and shown it to also be clonal, suggesting there may be occasional pollination occurring in the wild population and, for this individual at least, any morphological differences from the wild plants are not due to genetic differences.
Eucalyptus polybractea is polyphyletic in the phylogeny, although relationships among populations are not fully resolved, especially between the type population at West Wyalong (samples PSF74A and PSF74J) and the Victorian goldfields populations (samples PSF21C, PSF27A and PSF27H). Although our phylogenetic analyses suggest that the two samples from the Wimmera (samples PSF56B and PSF56C) are potentially conspecific with E. wimmerensis and the West Wyalong population is sister to the rest of the core E. odorata complex ( Fig. 5 View Fig ), in our network analyses collections from the Victorian goldfields and West Wyalong were more genetically similar to one another, and to E. yarriambiack and E. filiformis , than to the Wimmera samples ( Fig. 2 View Fig ). On the basis of the supported Flinders Ranges clade, which contains both E. polybractea and E. cajuputea samples ( Fig. 5 View Fig ), we suggest that E. polybractea should not be considered to occur west of the Murray Basin.
Although relationships among E. wimmerensis samples in the phylogeny remain unclear, they show little genetic differentiation, although the species’ boundaries remain unclear, given the uncertainty regarding the identity of the E. polybractea populations in the Wimmera. Although E. wimmerensis subsp. grata and Eucalyptus walshii were not genetically distinct from E. wimmerensis in our analyses, the PCA ( Fig. 3 View Fig ) and ABBA-BABA tests ( Table 4 View Table 4 ) suggested a low level of introgression from E. microcarpa , which may be responsible for the more robust stature of plants in these populations. The other species found only in the Wimmera and adjacent areas of SA, namely E. silvestris and E. hawkeri , both appear to represent more recent geneflow between E. wimmerensis and the co-occurring E. microcarpa on the basis of our ABBA-BABA and NewHybrid tests ( Table 4 View Table 4 ). This largely fits with the classifications of both Nicolle (2019) and Brooker et al. (2015), although those authors suggested E. odorata as being the E. odorata complex parent in the case of E. silvestris . We have some hesitancy ruling out this hypothesis, given our sampling, because we have shown that there is little genetic distinction between E. odorata and E. wimmerensis , and we cannot rule out there being populations in the Wimmera or adjacent areas of SA that better fit in E. odorata that we have not sampled.
The distinctions between the western taxa, E. cajuputea , E. odorata , and South Australian populations of E. polybractea , are unresolved in our study and require further investigation; however, it is clear what we have called E. polybractea in the Flinders Ranges has minimal genetic links to the typical E. polybractea of Victoria and NSW and may be best considered conspecific with E. cajuputea . Samples from the Flinders Ranges, identified as both E. cajuputea and E. polybractea , form a single clade in our phylogeny when the two aberrant samples are excluded ( Fig. 5 View Fig ), suggesting that there is a single lineage in this region that is distinct from other populations from west of the Murray Basin. The two aberrant samples, one each identified as E. polybractea and E. cajuputea , showed significant negative results for most comparisons in our ABBA-BABA tests ( Table 4 View Table 4 ), suggesting that introgression from a species we have not sampled is possible. The only other E. section Adnataria species that occur at Wilpena Pound and with which hybridisation may be occurring are E. porosa , which we have sampled and can therefore rule out, and E. intertexta R.T.Baker , which we have not sampled as part of this study. Eucalyptus intertexta ( E. series Buxeales ) is related to E. populnea and E. largiflorens , potentially explaining the single positive D -statistic with E. populnea in our ABBA-BABA tests, although the unresolved placement of the E. populnea sample in our phylogenies confounds this because we are unable to establish the relationship between this species and other members of E. series Buxeales . The mallee species E. leptophylla also co-occurs with the population these samples were sourced from and is superficially morphologically similar to members of the E. odorata complex, despite being in E. section Bisectae . This species may be the unknown parent, with its comparatively distant relationship to the E. odorata complex potentially explaining the significant negative D -statistics. Although we have a single sample of this species in our dataset, as part of the most divergent outgroup clade, we were not able to use it as an ingroup in ABBA-BABA tests to test this hypothesis because these require the inclusion of an outgroup with an evolutionary divergence point prior to the divergence of the three ingroup samples ( Durand et al. 2011). The other samples of E. cajuputea and E. odorata formed a polytomy along with the Flinders Ranges clade in the ML analysis, which may support a lack of distinctness of these two species outside the Flinders Ranges.
Genetic variation within the core E. odorata View in CoL complex as a cline
Our findings of extensive introgression among members of the E. odorata complex and co-occurring box species is not unexpected given that previous studies on E. section Adnataria have shown extensive hybridisation leading to morphological taxa not forming genetic clades ( Flores-Rentería et al. 2017). However, in regard to the taxonomy of the E. odorata complex, the nature of the core of the clade as a discontinuous cline of morphological and genetic variation running from the Flinders Ranges, south and then east through south-eastern SA, east through the Wimmera and then the Goldfields of Victoria and then north to West Wyalong in NSW also plays a significant role in disagreements among authorities over where taxonomic boundaries should be drawn. This clinal genetic variation has been taxonomically divided by different authors at different points on the basis of different factors they consider most important for classification and, in this paper, we have, a priori, broken it into the seven largely geographically distinct species ( E. odorata , E. cajuputea , E. wimmerensis , E. polybractea , E. filiformis , E. walshii and E. yarriambiack ).
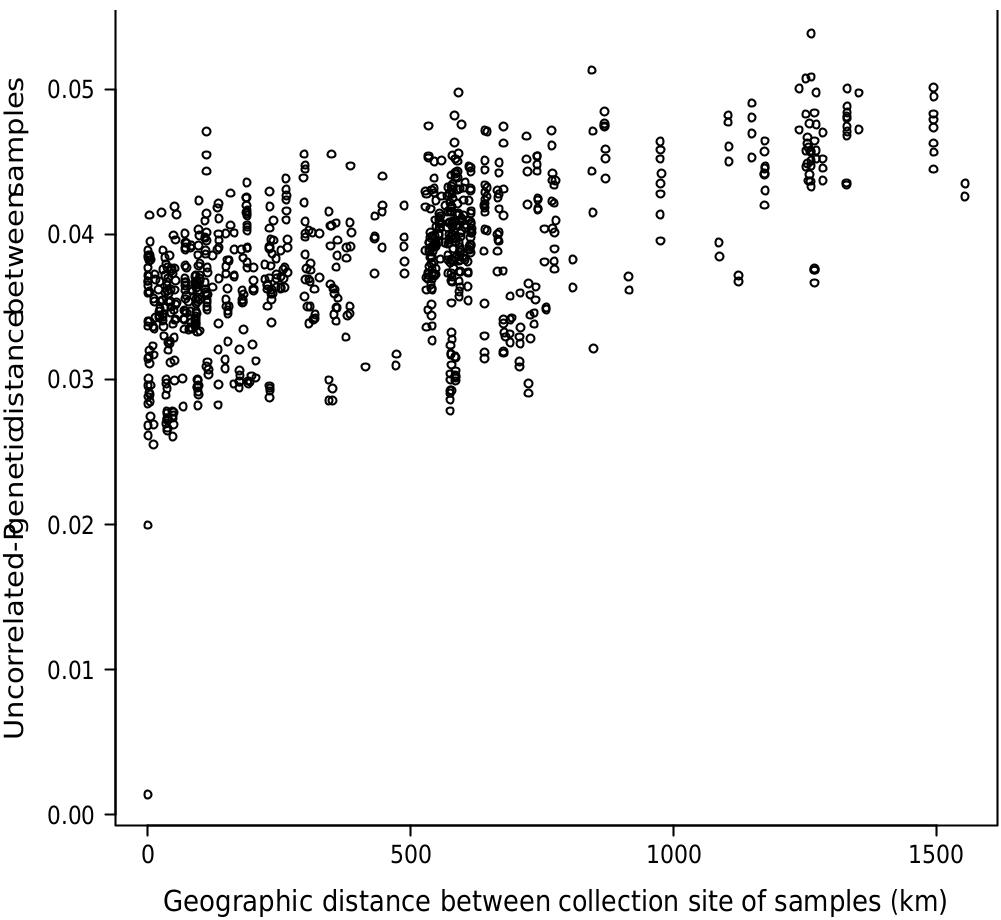
We see evidence for this cline in the most distal populations (Flinders Range and West Wyalong) being the most distinct, and in relationships between neighbouring populations fluctuate between analyses. This includes, for instance, E. filiformis clustering with E. wimmerensis in one network ( Fig. 2 b View Fig ) but being more closely related to E. polybractea from the Victorian goldfields in other analyses ( Fig. 2 a View Fig , 5), and the swapping of relationships between the three main groups (samples from west of the Murray River Basin, E. wimmerensis and allied samples, and eastern E. polybractea and allied samples) in the core group between analyses, even if the alternate relationships are not supported ( Fig. 4 View Fig ). This may be indicative of recent rapid diversification of a widespread ancestral population that has undergone vicariance at multiple locations at approximately congruent times. Clines along which diversification has occurred, often resulting in morphologically distinct species with a hybrid zone between them, have previously been observed in other eucalypt groups, including E. populnea and E. brownii Maiden & Cambage ( Holman et al. 2003), E. melanophloia F.Muell. and E. whitei Maiden & Blakely ( Holman et al. 2011), and the green ashes ( E. sect. Eucalyptus ; Rutherford et al. 2018). The strong support for IBD ( Fig. 6 View Fig , r: 0.597) in our data is congruent with the existence of a genetic cline.
Taxonomy within the E. odorata View in CoL complex
With the extensive level of interspecific geneflow, our dataset has provided evidence for, and the lack of resolved relationships among populations in the E. odorata complex; we do not feel that it is appropriate to make major taxonomic changes on the basis of our study without further work to investigate patterns of diversity in finer detail. However, we see several approaches that could be taken to clarify the taxonomy of the group as outlined in Table 6 View Table 6 . The simplest is to uphold the current morphology-based species classification, with adjustments to species distributions where necessary to match resolved phylogenetic relationships. This approach, essentially applying the morphological species concept, would require accepting the recognition of hybrid entities as species, which is being realised as a major driver of plant evolution ( Mayr 2000). The one clear change that is well supported in our data is that populations currently regarded as E. polybractea in the Flinders Ranges are not related to the eastern populations of that species and the circumscription of E. cajuputea should be expanded to include these populations.
The second approach is to greatly reduce the number of species in the complex to only those that are supported as monophyletic, strictly applying the phylogenetic species concept ( Baum 1992). This approach would likely reduce the complex back to two or three species, depending on what future study reveals regarding the distinctness of the northern populations of E. viridis . Eucalyptus aenea and E. castrensis would be synonymised with E. viridis and the cline of the rest of the complex broken up as follows: E. odorata to include populations west of the Murray Basin, E. wimmerensis to cover all populations in the western Wimmera of Victoria and adjacent areas of SA, and E. polybractea to accommodate populations from the eastern Wimmera, Victorian goldfields and around West Wyalong. Additionally, the name E. viridis var. latiuscula may need to be resurrected and its taxonomic rank re-assessed to accommodate the northern E. viridis populations that may represent a further extension of this cline. We would advocate that this approach is less than optimal, because each of these taxa would cover a large range of morphological variation that is somewhat correlated with geography and there are outstanding questions regarding the monophylly of these taxa. For these reasons, at this point, we would advocate a looser application of the phylogenetic concept and using the framework of integrative taxonomy to consider both the morphological and molecular evidence, synonymising species with molecular evidence against them being distinct entities, while maintaining morphological taxa with inconclusive molecular support for their status as distinct populations.
Following this reasoning, E. hawkeri and E. silvestris may need to be synonymised because both represent intergrades between E. wimmerensis and E. microcarpa . Although also showing introgression from E. microcarpa , E. walshii is perhaps better synonymised with E. wimmerensis , given its much closer genetic ties to this species. Recognition of E. filiformis is problematic, because it is clonal and, given its close placement to E. polybractea in our phylogeny and networks ( Fig. 2 View Fig , 5), possibly an outlying population of E. polybractea that has unique morphology owing to the small population size causing bottlenecking and genetic drift. A similar problem exists for E. yarriambiack because our data suggest that it is not experiencing introgression from the co-occurring E. largiflorens , but does not represent a distinct lineage from E. polybractea , rather being another potential small, isolated population undergoing genetic drift or a genetic bottleneck that should be synonymised with this species. However, in the case of E. yarriambiack , the population is not clonal, holds greater genetic diversity than does the clonal E. filiformis and is far enough outside the range of E. polybractea that there is likely to be no ongoing gene flow with populations of that species, all suggesting that the species status for E. yarriambiack is not unreasonable. For E. viridis , E. aenea and E. castrensis , we recommend that further phylogenetic studies are undertaken before taxonomy is re-assessed, because, although we have shown these three taxa are each other’s closest relatives, we have not sampled widely enough to determine whether E. aenea and E. castrensis are distinct lineages from E. viridis or isolated populations experiencing introgression from the co-occurring and more locally abundant grey-box species E. albens . In addition, further work is needed to investigate the relationships of the Queensland populations currently regarded as E. viridis to that species and to E. polybractea , or whether the name E. viridis var. latiuscula needs to be resurrected and given the rank of species.
| MP |
Mohonk Preserve, Inc. |
| ML |
Musee de Lectoure |
| J |
University of the Witwatersrand |
| E |
Royal Botanic Garden Edinburgh |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |