Etheostoma sitikuense Blanton, 2008
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1963.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03A37727-FFBB-FFCC-7284-BBEDFB6EFE51 |
|
treatment provided by |
Felipe |
|
scientific name |
Etheostoma sitikuense Blanton |
| status |
sp. nov. |
Etheostoma sitikuense Blanton View in CoL , new species
Citico Darter
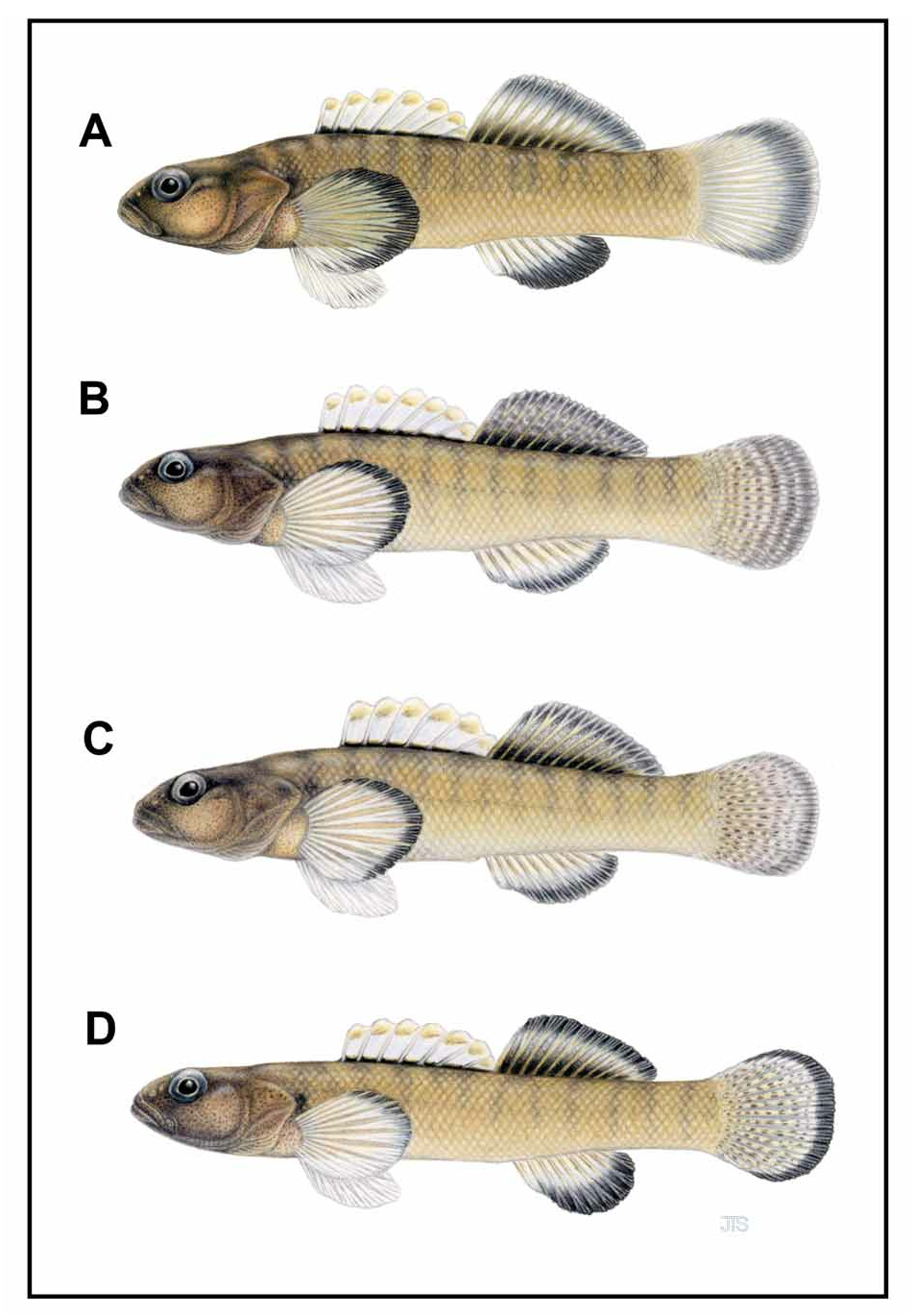
( Fig. 4c View FIGURE 4 )
Holotype. UF 172574, male, 43.7 mm SL, Citico Creek at pool just above bridge at Cherokee Forest Service Boundary , Monroe County, Tennessee, 16 May 1992, J. Shute, P. Rakes, and R. Biggins.
Paratypes. Tennessee River drainage— Little Tennessee River system.
Tennessee: Monroe County: TU 191558 (2, 32.9–50.2 mm SL) Citico Creek, 4.8 km east of Tariffville , off Citico Creek Rd , (35.508056° N, 84.104722° W) GoogleMaps , 9 April 2000, K. Piller, H. Bart, and J. Tipton; UF 172574 (2, 31.6–33.6 mm SL) same locality GoogleMaps ; USNM 394526 About USNM (2), Citico Creek at river km 9.5 , 12 Feb. 1983, G. Dinkins and C. Dinkins; UT 91.2558 (1), same locality .
Additional material (nontypes).
Tennessee River drainage—Little Tennessee River system
Tennessee: Blount Co.: Abrams Creek: UMMZ 129475 (2, 0, 0); UMMZ 201881 (1, 1, 1); Monroe Co.: Citico Creek: INHS 78168 (1, 0, 1); NCSM 30728 (8, 0, 3); UT 91.1917 (2, 0, 2); UT 91.4573 (2, 4, 2).
Diagnosis. Etheostoma sitikuense is distinguished from all members of the E. percnurum species complex by more pored lateral-line scales (34 vs. 28 or fewer); intermediate anal-fin band width (range = 33–39% vs. 49–58% in E lemniscatum and E. percnurum and 29–33% in E. marmorpinnum ); fewer transverse scale rows (15 vs. 16); shorter pectoral (P1L, =240 vs. 245 or greater) and pelvic (P2L, =180 vs. 197 or greater) fins; and wider (BW, =80 vs. 69 or less), deeper (BD1, =178 vs. 173 or less and BD2, =158 vs. 153 or less) body. E sitikuense is further distinguished from E. percnurum and E. lemniscatum by narrower distal band on the second-dorsal fin (range = 9–16% of fin height vs. 23–25%). From E. percnurum and E. marmorpinnum by distal caudal-fin band width (range = 15–18% of fin length vs. 12–15% in E. marmorpinnum and 17–25% in E. percnurum ); percentage of area along first-dorsal fin base scaled (70% vs. 20% in E. percnurum and 100% in E. marmorpinnum ); and intermediate number of scales around caudal peduncle (24 vs. 23 or 25, respectively). From E. lemniscatum and E. marmorpinnum by fewer lateral-line scales (43 vs. 44). From E. percnurum by diffuse marbling or stippling in medial portion of second dorsal fin of nuptial males (vs. uniformly dusky); tessellations in medial portion of caudal fin of nuptial males (vs. uniformly dusky); narrower distal band on pectoral fin (range = 14–20% vs. 29–32%); and fewer caudal-fin rays (15 vs. 18); and from E. marmorpinnum by lower percentage of the belly covered by scales (10% vs. 60–80%). Means of other measurements were also informative ( Table 9).
Description. Tables 1–7 provide meristic counts for most variables. Scales below lateral line 7–9 (8, =8.3±0.6); scales above lateral line 5–8 (6, =6.3±0.8); caudal peduncle scales below lateral line 8–12 (11, =10.5±1.1); caudal peduncle scales above lateral line 8–11 (10, 11, =9.9±1.4). Cheek, opercle, nape and breast devoid of scales. Branchiostegal rays six; gill membranes narrowly to moderately joined. First dorsal fin spines 6–7 (6, =6.4±0.5); second dorsal fin rays 11–13 (12, =12.0±0.4); pectoral fin rays 11–13 (12, =12.3±0.6); 2 anal spines; and anal rays 7–8 (8, =7.6±0.6). Preopercular-mandibular pores usually 10, rarely 8 or 11 (10, =9.9±0.6); infraorbital pores usually 6, rarely 5 (6, =5.9±0.3); anterior infraorbital pores 4 (4, =4.0±0.0); posterior infraorbital pores usually 2, rarely 1 (2, =1.9±0.3); supraorbital pores 4 (4, =4.0±0.0); supratemporal pores usually 4, rarely 2 (2, =3.9±0.5); left supratemporal pores usually 2, rarely 1 (2, =1.9±0.3); right supratemporal pores usually 2, rarely 1 (2, =1.9 ±0.3).
Measurements for males (nuptial and non-nuptial) and females are presented separately; male measurements are presented in Table 9. Females (n=5): SL 30.6–34.0 (=32.2±1.3); GW 80–100 (=86±9); IOW 50–60 (=52±4); HW 120–140 (=128±8); HL 300–340 (=322±15); P1L 260–280 (=270±10); P2L 200–230 (=214±11); D1H 110–160 (=132±19); D1L 160–190 (=172±16); D2H 130–170 (=154±18); AFH 130–150 (=138±11); CFL 210–240 (=226±11); BW 70–80 (=74±5); H1–H2 100–120 (=116±9); H1–H3 220–240 (=228±11); H1–B4 370–400 (=382±13); H1–H5 110–140 (=126±11); H1–B6 290–310 (=302±8); H2–H5 110–140 (=122±11); H3–B6 170–180 (=178±4); B4–B6 or BD1 160–200 (=178±18); B4–B7 240–290 (=276±21); B4–B8 310–320 (=316±5); B6–B7 360–390 (=378±13); B6–B8 310–340 (=326±15); B7–B8 or BD2 140–160 (=146±9); B7–B9 or D2L 190–230 (=208±16); B7–B10 200–220 (=210±7); B8–B9 220–270 (=240±20); B8–B10 or AFL 150–160 (=152±4); B9–10 110–140 (=118±13); B9–C11 140–170 (=154±13); and B10–C11 200–220 (=212±8).
Coloration and pigmentation in-life and preserved generally as described for E. percnurum (Jenkins 1994) . However, medial portion of second dorsal fin of nuptial males with diffuse, stippling to diffuse marbling and medial portion of caudal fin with distinct tessellations; tessellations confined to rays, not forming bands; distal band on anal (range = 33–39% of fin height), second dorsal (range = 9–16% of fin height), caudal (range = 15–18% of fin length), and pectoral fins (range = 14–20% of fin length) narrower than in E. percnurum . For all individuals, number of transverse bars for males rarely 0 (poorly developed), usually 11–13 (12, =6.0±6.6), for females 11–13 (12, =11.9±0.7); number of dorsal saddles for males rarely 0, usually 7 (7, =4.7±3.6), for females rarely 0, usually 7–8 (7, =6.3±2.8); number of tessellations along medial caudalfin ray for males rarely 0, usually 5–7 (0 and 5, =3.8±3.1), for females 5–7 (6, =5.9±0.7); and caudal peduncle of nuptial males with 1 caudal spot, 2 diffuse spots, or no obvious spot.
Distribution. The Citico Darter occupies an approximately 3.5 river km reach of Citico Creek in Monroe County, Tennessee, just downstream of a U.S. Forest Service boundary. The creek is a tributary of Tellico Lake, an impoundment of the mainstem Little Tennessee River. The population in Citico Creek historically extended further downstream than its current distribution suggests. One individual was collected 13 December, 1979 from lower Citico Creek prior to its inundation by Tellico Lake (D. Etnier, pers. comm.). The darter is historically extirpated from Abrams Creek, a tributary of Chilhowie Lake also impounding the Little Tennessee River, in Great Smoky Mountains National Park, Blount County, Tennessee, where it is known from three specimens collected in 1937 and 1940. This and other at-risk fish species ( Jenkins & Burkhead 1984; Simbeck 1990) apparently were extirpated from Abrams Creek by application of rotenone throughout the tributary system below Abrams Falls during 1957, a plan designed to reduce food and habitat competition for a Rainbow Trout fishery ( Lennon & Parker 1959). Etheostoma sitikuense has been propagated and reintroduced to lower Abrams Creek, below Abrams Falls and stocked in Tellico River using Citico Creek stocks ( Rakes & Shute 2005; Shute et al. 2005; Rakes & Shute 2008).
Ecology. Abrams Creek (Blue Ridge Province) and Citico Creek (Blue Ridge and Ridge and Valley) are moderate-sized streams that are characterized by alternating riffles, runs, and pools with cobble and small boulders. In Citico Creek, nests and nest-guarding by nuptial males have been observed beneath slab-rocks in the margins of pools and in swifter runs ( Rakes et al. 1992). Abrams Creek is divided by Abrams Falls at rkm 23.5, which divides the aquatic communities into two distinct portions ( Simbeck 1990). Etheostoma sitikuense was known to occur below the falls in the lower reaches of the mainstem of Abrams Creek; the few records prior to extirpation are known only from several kilometers upstream of the confluence with the Little Tennessee River. Etheostoma flabellare occurs above the falls, and it appears that the two were largely parapatric in Abrams Creek. Etheostoma sitikuense is the only known Catonotus in Citico Creek. There are no known historic records of E. sitikuense from the Tellico River, but the species was recently stocked (using Citico Creek individuals as stock; Rakes & Shute 2008) downstream of the National Park boundary to TN Hwy 360 bridge. In Tellico River E. sitikuense is parapatric with a unique, but undescribed form of E. flabellare found above the falls on the upper Tellico River inside the park boundary ( Blanton 2001).
Conservation Status. Known threats to Abrams and Citico Creek include agricultural runoff, sedimentation due to bank erosion, and poor land use practices. For example, nearly the entire reach of Citico Creek occupied by E. sitikuense flows through privately owned property where streamside habitat and buffer zones are not monitored or regulated. Etheostoma sitikuense may represent the most stable member of the E. percnurum complex because it is now found in three streams of the Little Tennessee system, although the populations are separated by large mainstem impoundments and cold tailwaters. The stocked, reintroduced population in Abrams Creek appears stable; recruitment has been observed since 1995 ( Shute et al. 2005; Rakes & Shute 2008). The status of the stocked Tellico River population is not known, but the species appears to be moderately abundant in the small reach occupied in Citico Creek ( Shute et al. 2005). However, the extremely limited distribution of E. sitikuense and the known extirpation of past populations point to the need for federal protection. Continued monitoring of habitat quality, land use practices, and population status are recommended. A recovery plan that focuses on these factors and includes goals to alleviate impacts to these stream reaches is needed. While continued propagation may be beneficial to the long-term survival of the species, further translocation outside the species known native range is not recommended. The distribution of the introduced E. sitikuense population in Tellico River should be closely monitored to ensure it does not encroach on the distinct, isolated population of Fantail Darter occurring above the falls in the upper Tellico River.
Etymology. The name ‘ sitikuense’ comes from the Cherokee Indian word ‘sitiku’ for a place of clean fishing water and is the origin for the name of Citico Creek. Citico Darter refers to the type locality of the species, where the only extant, non-introduced or propagated population of this species occurs.
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
| R |
Departamento de Geologia, Universidad de Chile |
| TU |
Tulane University, Museum of Natural History |
| UMMZ |
University of Michigan, Museum of Zoology |
| INHS |
Illinois Natural History Survey |
| NCSM |
North Carolina Museum of Natural Sciences |
| UT |
University of Tehran |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |