Ctenophorus mirrityana, McLean & Moussalli & Sass & Stuart-Fox, 2013
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.65.2013.1600 |
|
persistent identifier |
https://treatment.plazi.org/id/03EB87BA-6A16-FFF3-FC56-B58CE7C2E7ED |
|
treatment provided by |
Carolina |
|
scientific name |
Ctenophorus mirrityana |
| status |
sp. nov. |
Ctenophorus mirrityana sp. nov.
Barrier Range Dragon
Figs 3A View Fig , 7 View Fig , 8 View Fig , 9A View Fig
Holotype. AMS R 47295 ( Fig. 8 View Fig ), an adult male with label data: Australia, New South Wales, Mootwingee [Historic Site, Mutawintji National Park], 31°17'S 142°18'E, 20 January 1975, collector P. Rankin et al. [Office of Environment and Heritage]. GoogleMaps
Paratypes. All specimens are from New South Wales. AMS R 14661, Mootwingee Waterholes (31°19'S 142°19'E) GoogleMaps ; AMS R45527–9 , AMS R 47294, AMS R 47298, AMS R 47335, AMS R 61514, AMS R 68792, AMS R125297 , AMS R133122–3 , AMS R145339 , AMS R145341 , AMS R145593 , AMS R146252–3 , AMS R149014 , AMS R149021 , AMS R149143 , AMS R149146–7 , AMS R151011–2 , AMS R151014–7 , AMS R151019–20 , AMS R151733–5 , AMS R153361 , AMS R154857 , AMS R154859 , AMS R154863–4 , AMS R154869–70 , AMS R154872 , AMS R154932–8 , AMS R157300–7 , AMS R157317–23 , AMS R157325–8 , AMS R157330–40 , AMS R157342 , AMS R157344 , AMS R157346–9 , SAM R5194 A–B , SAM R 14468A–B , SAM R 31655 , NMV D11511 , NMV D11770 , NMV D18019 , NMV D40134 View Materials –5 About NMV , NMV D50516 , NMV D56318 View Materials –22 About NMV , Mootwingee National Park (31°17'S 142°18'E) GoogleMaps , AMS R107358–67 , 6 km S Mootwingee National Park (31°18'S 142°15'E) GoogleMaps , AMS R161707–8 , Homestead Gorge, Mootwingee National Park (31°16'35"S 142°18'5"E) GoogleMaps , NMV D56323, Broken Hill (31°58'S 141°27'E) GoogleMaps , AMS R 50540, Koonenberry Mountain (30°31'S 142°18'E) GoogleMaps , AMS R168437 , “ Belmont Station ”, N Silverton (31°46'11"S 141°14'33"E) GoogleMaps .
McLean et al.: A new dragon lizard from New South Wales 55
Diagnosis
A member of the Ctenophorus decresii species complex (Houston & Hutchinson, 1998), C. mirrityana sp. nov. is a moderately sized, seXually dimorphic, rock-dwelling dragon lizard with a strongly compressed head and body ( Fig. 7 View Fig ). Within the species compleX, C. mirrityana sp. nov. is distinguishable by the following combination of characters: head relatively small for body size; snout scales keeled or weakly wrinkled; vertebral scales flat and pale in colour; black lateral stripe from tympanum to groin; thinner, noncontinuous orange stripe within black lateral stripe; flanks lack tubercular scales; male throat coloration pale cream with parallel grey stripes and black central stripe sometimes overlain with orange flushes.
Description
A moderately sized dragon lizard reaching a maXimum SVL of approXimately 91 mm and total length of 266 mm. Head strongly compressed and small for body size (relative to other members of the species compleX; AppendiX 2); nostril located beneath a sharp canthus rostralis. Body and base of tail dorsoventrally flattened, allowing the species to squeeze into narrow rock crevices. Tail long and evenly tapered to a fine tip; forelimbs moderately long reaching or almost reaching groin when adpressed; hindlimbs long and reaching or almost reaching snout when adpressed, digits are long and slender; finger lengths: 4> 3> 5> 2> 1; toe lengths: 4> 3 ≥ 5> 2> 1.
Characteristic of the genus Ctenophorus , a row of enlarged, keeled scales eXtends from the nostril, below the eye to above the tympanum (Houston & Hutchinson, 1998). Scales on snout are keeled to lightly wrinkled; eyelid fringed with row of acute scales; 14−19 supralabial and infralabial scales; 4−6 scales between rostral and nasal; 4−6 scales between supralabial and nasal; 9−12 internasal scales; 21–27 subdigital lamellae on the fourth toe. The skin on the neck is loose, forming folds of skin above and behind the tympanum with small rows of pale coloured spines. A low nuchal crest of conical scales is present and terminates in line with the shoulders. Vertebral scales are flat and pale in colour and can be raised on a fold of skin during behavioural displays. Dorsal scales are smooth or very lightly keeled, becoming smaller laterally; flanks lack scattered tubercular scales. Scales on the dorsal surfaces of the limbs and tail are keeled. A strongly formed gular fold is present, eXtending across the shoulders. Ventral scales are around the same size as vertebral scales, larger than dorsal and lateral scales, flat and homogenous, with the eXception of the scales along the gular fold which are smaller. Thirty four to forty two evenly spaced femoral and preanal pores are arranged in a straight line along the thighs, interrupted medially by 7−9 scales. Pores are present but smaller in females.
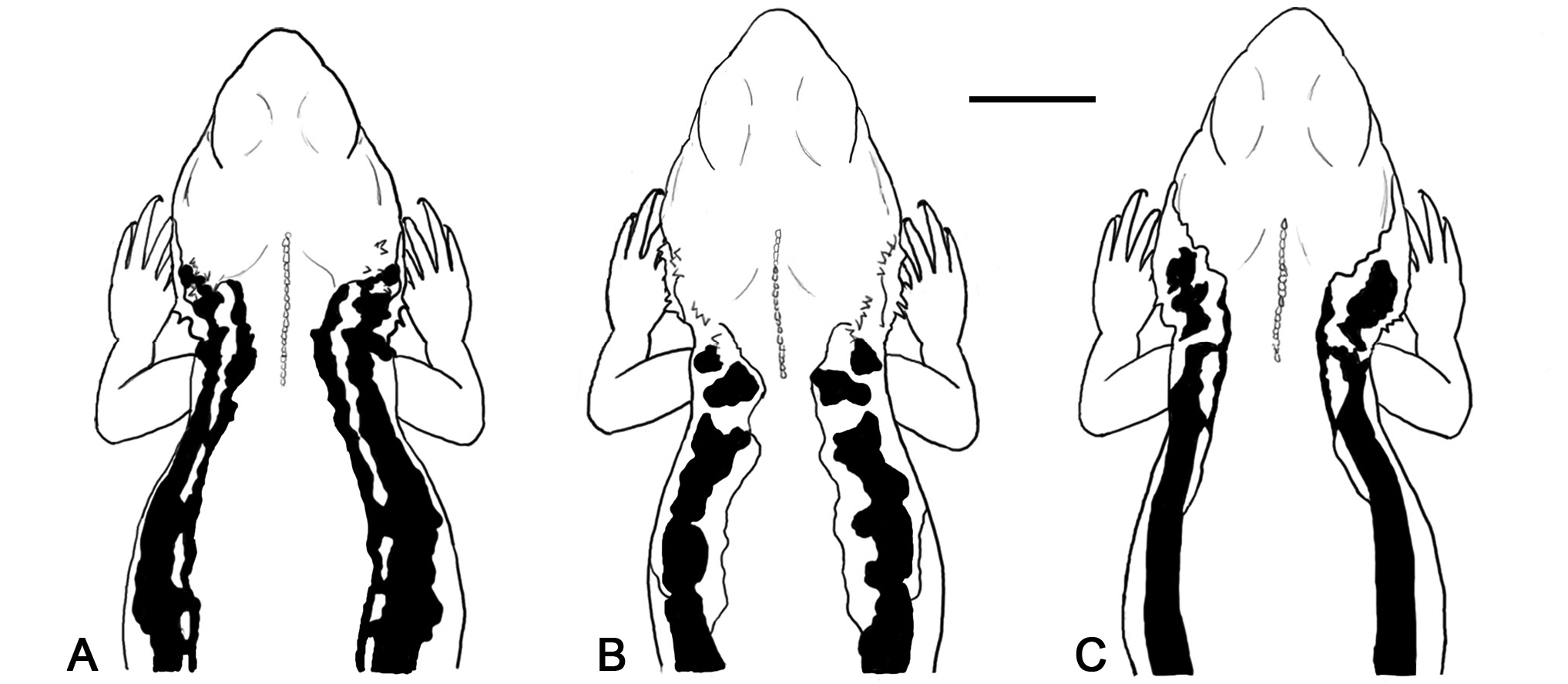
Adult male base colour varies from grey-blue to very pale blue which appears more blue when the lizard is warm ( Fig. 7A View Fig ). The vertebral line is pale, becoming more grey-blue towards the flanks, while the dorsal surfaces of the head, tail and hindlimbs are grey-brown. The head is orange around the eyes, nostrils and along the upper jaw, and beneath the tympanum to the neck; however, the eXtent and brightness of this coloration varies among individuals. A black lateral stripe begins posterior to the eye, becoming thicker posterior to the tympanum and terminating at the groin.A thinner, noncontinuous orange stripe, often bordered by pale blotches, begins at the tympanum and runs within the black lateral stripe to the groin ( Fig. 9A View Fig ). Pale blue coloration mottled with cream occurs beneath the lateral stripe and on the forelimbs. Ventrally males are white to cream with orange flushes on the belly, hindlimbs, and tail during the breeding season.A grey to black chest patch tapers to a point midbody and eXtends along the forelimbs in some individuals. Male throat coloration consists of cream base colour with parallel grey stripes along the length of the throat, often overlain with orange flushes around the snout, which may cover the whole throat in some individuals ( Fig. 3 View Fig ). A distinct black stripe runs along the mid line from gular fold to snout but varies in length and intensity among individuals.
Adult females are cryptically coloured with brown, grey, and terracotta speckling ( Fig. 7B View Fig ). Dorsally, scales are browner with a thin, pale vertebral line. A black lateral stripe coupled with a thin terracotta stripe runs laterally along the flank, although this may be less prominent than in males. Scales are greyer on the flanks below the lateral stripe. Ventrally females are white to cream with grey stripes on the throat and orange flushes on the belly during the breeding season. Juveniles resemble adult females in coloration and pattern but are often paler with more delicate speckling. Pattern remains clear on spirit preserved specimens; however, both males and females appear darker than in life and any orange coloration fades considerably.
Measurements (mm) and meristic counts of holotype. SVL, 76.87;AG, 32.99; TL, 142; HL, 25.38; HW, 17.60; HD, 10.96; EYE, 5.47; SL, 8.31; JL, 13.07; NW, 6.79; HUML, 11.48; RADL, 9.46; HAND, 13.46; FING, 8.31; FEML, 18.72; TIBL, 20.89; FOOT, 27.52; TOE, 14.14; SUPRA, 17; INFRA, 17; ROSNAS, 4; SUPRANAS, 5; INTERNAS, 10; SDL, 21; FP, 36.
Ecology and distribution
Ctenophorus mirrityana sp. nov. is a rock specialist, and occupies variable habitats ranging from scattered rock aggregates and road spoils, to rocky outcrops and gorges (Swan & Foster, 2005; Sass & Swan, 2010). Previous studies associated with this species have suggested that the percentage cover of eXposed rock outcropping, the presence of large rocks, and landscape position are the greatest influence of habitat occupancy (Sass & Swan, submitted). To date, C. mirrityana sp. nov. has been detected in mulga shrubland dominated by Mulga ( Acacia aneura ) and Dead Finish ( A. tetragonophylla ), black oak woodland dominated by Black Oak ( Casuarina pauper ) and Western Rosewood ( Alectryon oleifolius ), and hummock grass woodland dominated by Gum Coolibah ( Eucalyptus intertexta ) and Red Mallee ( E. socialis ) with an understorey of Porcupine Grass ( Triodia scariosa subsp. scariosa ; Swan & Foster, 2005; Sass & Swan, 2010).
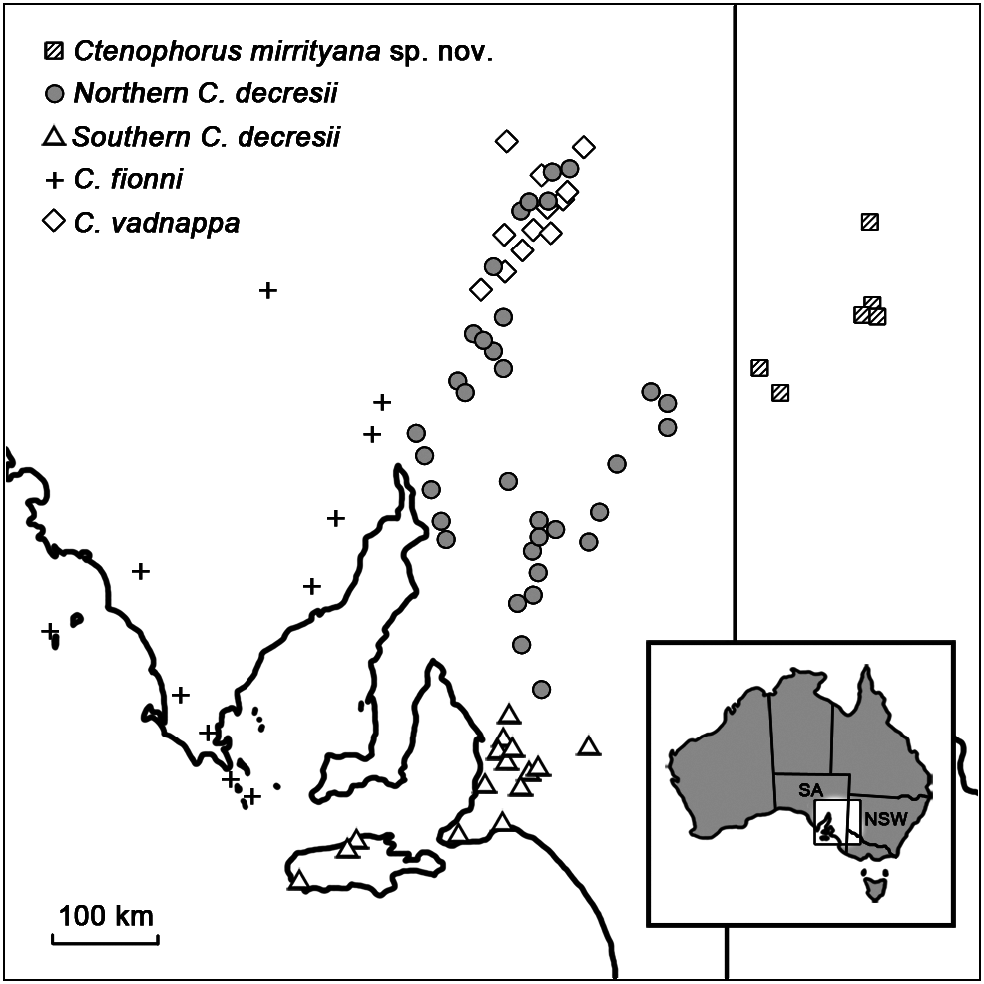
The species is active and conspicuous during hot weather and shelters in rock crevices when threatened or inactive. Males perform conspicuous courtship and territorial behaviour involving push-ups and tail-flicks, and will often perch in prominent positions during displays. Ctenophorus mirrityana sp. nov. is allopatric to all other members of the species group, and is currently known from four localities in western NSW. It has recently been recorded from Mutawintji National Park and adjacent properties (Swan & Foster, 2005), and the Silverton Wind Farm site, 35 km north west of Broken Hill (Sass & Swan, 2010). Additionally, museum specimens from Broken Hill and Koonenberry Mountain, north of Mutawintji National Park were collected in the 1970s ( Fig. 1 View Fig ).
Etymology. The specific epithet mirrityana is a word meaning “out in the sunlight” in the localAboriginal language (Paakantyi; Hercus, 1993), in reference to the conspicuousness of the species during hot weather. There are several rock engravings depicting lizards at Mutawintji National Park (McCarthy & Macintosh, 1962), some of which may represent this species given it’s prominence in the area. We propose Barrier Range Dragon as the species’ common name.
Comparison between species
Ctenophorus mirrityana sp. nov. strongly resembles C. decresii in coloration. In both species, male dorsal coloration consists of blue-grey base colour with a black lateral stripe and bright yellow-orange coloration around the head, however, throat coloration differentiates these species. The throat colour of C. mirrityana sp. nov. is cream with grey stripes, overlain with orange flushes, with a black central stripe. The black stripe is distinct to the species, although some northern C. decresii individuals may have a small, central black patch on their throat. Conversely, northern C. decresii males have orange, yellow, orange and yellow, or grey throats ( Teasdale et al., 2013) and southern C. decresii males have blue or blue and yellow throats ( Houston, 1974). Differences in lateral colour pattern further distinguish C. mirrityana sp. nov. and C. decresii ( Fig. 9 View Fig ). Ctenophorus mirrityana sp. nov., has a non-continuous stripe of orange coloration which runs within a black lateral stripe between the tympanum and groin ( Fig. 9A View Fig ). In southern C. decresii , the lateral stripe is “pinched” along its length by the margining yellow-orange coloration and is interrupted on the neck, forming a separate black blotch behind the tympanum ( Fig. 9B View Fig ). Conversely, the black lateral stripe of northern C. decresii is relatively straight edged and continuous, and a cream, yellow or orange stripe runs along its upper edge and generally terminates just posterior to the shoulder ( Fig. 9C View Fig ). In other aspects of morphology, the head of C. mirrityana sp. nov. is smaller (relative to SVL) than that of C. decresii , and C. mirrityana sp. nov. has fewer internasal scales, fewer femoral pores, a greater number of supralabial and infralabial scales, a prominent pale vertebral line, and lacks scattered white tubercular scales on the flanks.
Notable phenotypic differentiation eXists between C. mirrityana sp. nov. and the other members of the C. decresii species group. Ctenophorus mirrityana sp. nov. has a blue-grey body colour with a black lateral stripe compared with vertical orange-red and black flank markings in C. vadnappa , rows of pale spots in C. fionni , and a grey-brown body colour with pale lateral blotches forming vertical bars in C. tjantjalka . Male C. mirrityana sp. nov. have cream throat coloration with grey stripes, a black central stripe, and orange flushes compared with yellow and blue in C. vadnappa , cream and yellow in C. fionni , and cream with fine grey reticulations in C. tjantjalka . Furthermore, while C. mirrityana sp. nov. has a dorsoventrally flattened head and smooth or weakly keeled snout scales, C. tjantjalka has a relatively short and deep head and coarsely wrinkled snout scales ( Johnston, 1992). Snout scales are similarly wrinkled in C. vadnappa ( Houston, 1974) , which also has longer hindlimbs than C. mirrityana sp. nov. (Fig. 5). The distribution of C. mirrityana sp. nov. does not overlap with any other member of the group ( Fig. 1 View Fig ); however, it may abut the most eastern populations of northern C. decresii around the SA/NSW border. Consequently, C. mirrityana sp. nov. is most likely to be confused with northern C. decresii based on distribution.
The northern and southern lineages of C. decresii are further distinguishable from each other by coloration (as described above) and a combination of other morphological characters. Southern C. decresii is generally smaller, has fewer supralabial and infralabial scales, and a greater number of femoral pores than northern C. decresii . Furthermore, southern C. decresii individuals consistently have prominent white tubercular scales along their flanks, which are often absent in northern individuals.
Conservation status
The distribution of C. mirrityana sp. nov. is sufficiently restricted that it was (as C. decresii ) formerly recognized as endangered in NSW ( NSW Scientific Committee, 2002). The species distribution currently eXists as two disjunct populations approXimately 100 km apart; however, no field surveys have been undertaken in the intervening areas and C. mirrityana sp. nov. may be more widespread throughout the Barrier Range region than currently appreciated. Alternatively, these populations may be relicts of a previously wider distribution and under this scenario C. mirrityana sp. nov. may warrant Federal nomination as a threatened species under the Environmental Protection and Biodiversity Conservation Act (1999). Field surveys of other suitable sites are needed to determine the full distribution of the species to adequately assess its conservation status.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |