Creophilus galapagensis, 2011
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2011.00725.x |
|
publication LSID |
lsid:zoobank.org:pub:FBFE9195-BE04-4AFE-9417-6E38BCE6AB84 |
|
persistent identifier |
https://treatment.plazi.org/id/C3CC699D-2282-48C4-AB51-D67D50474AF1 |
|
taxon LSID |
lsid:zoobank.org:act:C3CC699D-2282-48C4-AB51-D67D50474AF1 |
|
treatment provided by |
Valdenar |
|
scientific name |
Creophilus galapagensis |
| status |
|
3. CREOPHILUS GALAPAGENSIS CLARKE View in CoL SP. NOV.
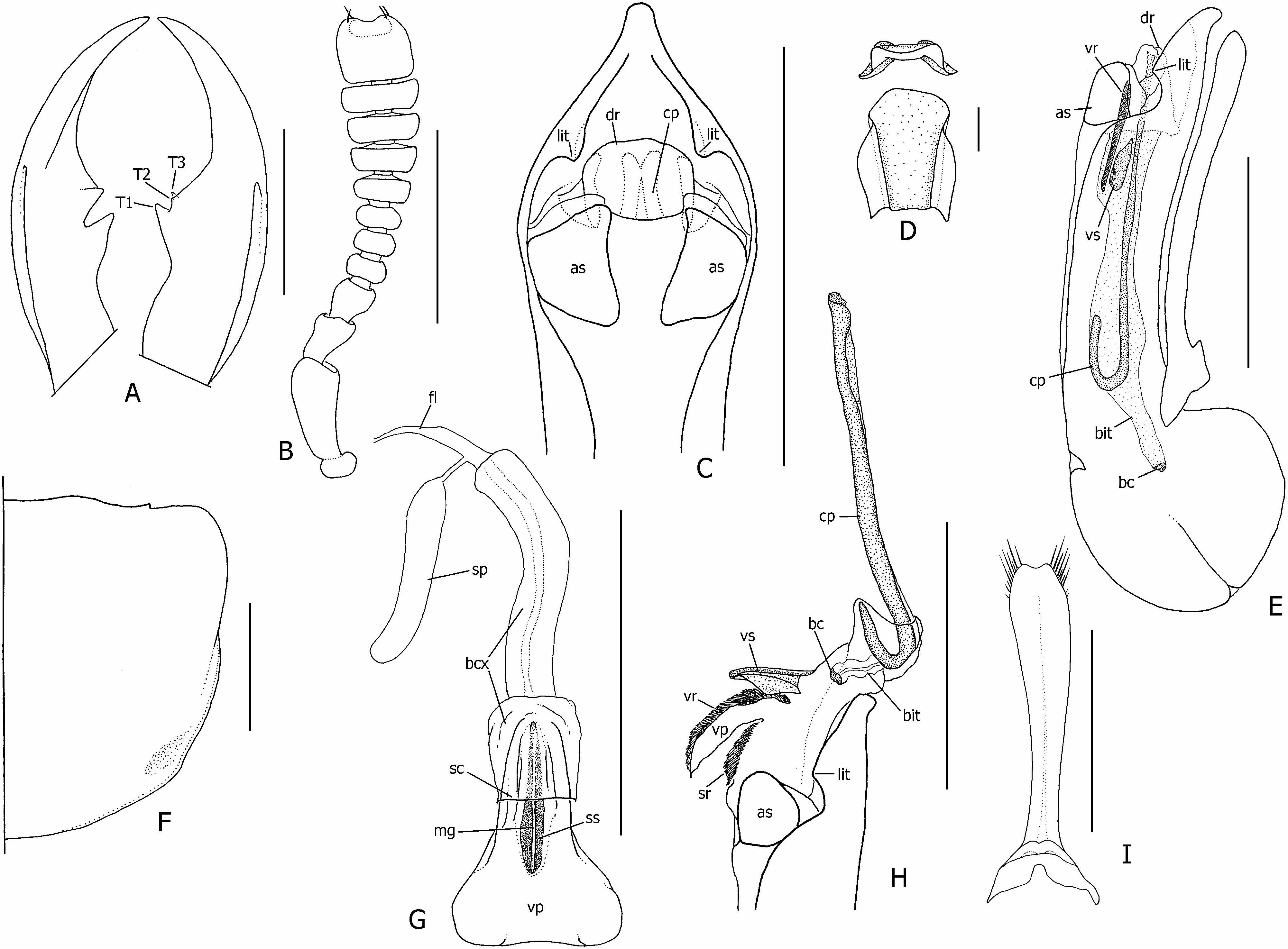
( FIGS 1H, 4D View Figure 4 , 18 View Figure 18 , 19A View Figure 19 , 20 View Figure 20 )
Type material: Holotype. ♂, ‘ ECUADOR: Galápagos:| Isla Isabela, Volcán| Alcedo (NE rim), 1100 m,| 0 °24′S, 91 °07′W,| 21–25.vi.1991 / shrub forest: FMHD#91-|203, Peck#91–246, carrion| traps, S. Peck| FIELD MUS. NAT. HIST./ FMNH-INS 0000 019 454/ [red] HOLOTYPE | Creophilus | galapagensis Clarke sp. nov. | designated by| D.J. Clarke 2010’ (in FMNH). The aedeagus and genital segments are dissected and stored in glycerin in a genitalia vial mounted with specimen. Paratypes (1069): all pinned specimens and alcohol lots with label ‘[yellow] PARATYPE | Creophilus | galapagensis Clarke sp. nov. | designated by| D.J. Clarke 2010’. 3 ♀, with labels ‘ISLAS GALAPAGOS| ISABELA| 12–11/85| lg. S. Abedrabbo/ ex.| AVE MUERTA [dead bird]/ 5/ Creophilus | maxillosus| (Linnaeus)| det. Newton 2003’: ‘ Creophilus | maxillosus| DET: S.Peck, 2002/ FMNH-INS 0000 019 470’; ‘FMNH-INS 0000 019 471’; ‘FMNH-INS 0000 019 472’ (in FMNH). 3 specimens, ‘Isabela| Cerro Azul [0 °54′S 91 °25′W]| [underside of label]SA 308–309/ Islas Galapagos| lg.H.Franz,V.-VI, 1975’: 2♂, ‘FMNH-INS 0000 017 435’; ‘FMNH-INS 0000 017 436’; 1♀, ‘FMNH-INS 0000 017 437’ (in NMW). 2 specimens, ‘Albermarle I. [ Isla Isabela]| Galapagos Is./ Cobes| Settlement| IV.24.06/ Coll. By F.X. Williams’: ♂, ‘FMNH-INS 0000 017 808’; ♀, ‘[in pencil] S.A.| 31/ FMNH-INS 0000 017 809’ (in CAS). 133 specimens, ‘ ECUADOR: Galápagos:| Isla Isabela, Santo| Tomás, 350 m,| 0 °51′S, 91 °02′W,| 6–9.vii.1985, mixed forest;| FMHD#85–1037,| Peck#85–214, carrion| traps, S. & J. Peck| FIELD MUS. NAT. HIST.’: 58♂ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 800’; 42♀ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 801’; 19♂ pinned, 8 with aedeagi dissected, FMNH-INS 0000 017 810– 828; 5♂ pinned, 4 with aedeagi dissected, FMNH-INS 0000 017 625–629; 9♀, [FMNH-INS 0000 017 616– 618; 829–834] (in FMNH). 1♂, ‘ECU.Galap.Isabela| Sto. Tomas, 300–350 m / 9–12.III.89, [Peck#]89–126| agric.z.carrion tp.| B.J Sinclair/ Creophilus | maxillosus| (Linnaeus)| det. Newton 2003/ FMNH-INS 0000 019 473’ (in FMNH). 1 ♂, aedeagus dissected on card, ‘Tagos [Tagus] Cove [0 °16′S 91 °22′W]| Albermarle I. [ Isla Isabela]| Galapagos Is./ IV-1–06/ Coll. by| F.X. Williams/ FMNH-INS 0000 017 807’ [parasitic worm in genitalia vial] (in CAS). 3 ♂, all with aedeagi dissected, ‘Villamil [0 °56′S 91 °1′W]| Albermarle I. [ Isla Isabela]| Galapagos Is./VIII-22– 06[1906]/ Coll. by| F.X. Williams’: ‘FMNH-INS 0000 017 804/ Creophilus | villosus| (Grav)’; ‘FMNH- INS 0000 017 805/ [in pencil] S.A. 44.’; ‘FMNH-INS 0000 017 806/ [in pencil] S.A.| 46.’ (in CAS). 5 specimens, ‘ ECUADOR: Galápagos I.| Isla Isabela, Volcán| Alcedo, crater rim [0 °25′S, 91 °6′W], 1000| m, 2.IV.1996, hand coll-| ecting, S. Peck 96–84| FIELD MUS. NAT. HIST.’, and with det. label ‘ Creophilus | maxillosus | (Linnaeus)| det. Newton 1998’: ♂, with aedeagus dissected ‘FMNH-INS 0000 017 416’; 4♀ [measurements taken, FMNH-INS 0000 017 417–420] (in FMNH). 779 specimens, same data as holotype: 349♂ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 802’; 381♀ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 803’; 5♂ pinned [measurements taken, FMNH-INS 0000 017 620–624]; 23♂ pinned, [FMNH- INS 0000 017 857–879]; 1♂ pinned, [FMNH-INS 0000 019 440]; 13♀ pinned, [FMNH-INS 0000 019 441–453]; 5♂ pinned, with aedeagi dissected, [FMNH-INS 0000 019 455–459]; 1♂ pinned, ‘FMNH-INS 0000 019 460’ (in FMNH); 1♂, 1♀ pinned, deposited in each of AMNH, BMNH, BPBM, CAS, CDRS, CMNC, IRSNB, MCZ, NMW, QCAZ, SEMC, and USNM. 1♂, same locality and habitat data as holotype, ‘... pitt &| bottle tps under| shrubs, SPeck, 91–245/ Creophilus | maxillosus| (Linnaeus)| det. Newton 2003/ FMNH-INS 0000 019 475’ (in FMNH). 22 specimens, ‘ ECUADOR: Galápagos:| Isla Isabela, Volcán| Alcedo (NE slope), 850 m,| 0 °22′S, 91 °06′W,| 20–25.vi.1991 / open forest: FMHD#91-|201, Peck#91–243, carrion| trap, S. Peck| FIELD MUS. NAT. HIST.’: 10♂, [FMNH-INS 0000 017 835–844]; 12♀, [FMNH-INS 0000 017 845– 856] (in FMNH). 1♂, same locality and habitat data, ‘... night| colln, S. Peck, 91–244/ Creophilus | maxillosus| (Linnaeus)| det. Newton 2003/ FMNH-INS 0000 019 474’ (in FMNH). 14 specimens, ‘ ECUADOR: Galápagos I.| Isla Isabela| Cerro Azul,| 5 km NE Caleta Iguana [0 °56′S, 91 °27′W],| 400 m, pampas;/ 20–25.V.1991, carrion| trap, S. Peck 91–154| FIELD MUS. NAT. HIST.’: 11♂, [FMNH-INS 0000 017 421– 431]; 3♀, [FMNH-INS 0000 017 432–434; -433 and -434 with measurements taken] (in FMNH). 89 specimens, ‘ ECUADOR: Galápagos I.| Isla Isabela, Volcán Cerro| Azul, 7 km NE Caleta| Iguana, 700 m,| 0 °56′S, 91 °26′W./ 20–25.V.1991, pampas;| FMHD#91–181, Peck#91-|155, carrion trap, S. Peck| FIELD MUS. NAT. HIST.’: 41♂ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 798’; 43♀ in 70% alcohol, with lot ID number ‘FMNH-INS 0000 017 799’; 2♂ pinned, ‘FMNH-INS 0000 017 794’; ‘FMNH-INS 0000 017 795’; 2♀ pinned, ‘FMNH-INS 0000 017 796–797’; 1♀ pinned, with measurements taken, ‘FMNH-INS 0000 017 619’ (in FMNH). 6 specimens, ‘Isabela| Umg. Sta. Tomas [in area of Santo Tomás]| [underside of label]SA 299/ Islas Galapagos| lg.H.Franz,V.-VI, 1975’: 3♂, [FMNH-INS 0000 017 438–440]; 3♀, ‘FMNH-INS 0000 017 441/ Creophilus | villosus Grav. | det.H.Franz’; ‘FMNH- INS 0000 017 442–443’ (in NMW).
Diagnosis: With characters of the maxillosus -group; integument and elytra uniformly pitchy black; elytral vestiture entirely black ( Fig. 1H); elytra rugose; abdominal microsculpture imbricate ( Fig. 19A View Figure 19 ); tergal chaetotaxic formula 2-2-2-2-2-4.
Description: Measurements (N = 10♂, 10♀). Forebody length: ♂ 5.3–8.7 mm, ♀ 5.4–7.5 mm. See supporting Table S4 for comparison of ranges of male and female ratios. Head. Head subtrapezoidal to subrectangular, widest posteriorly; large males with slight hind angles evident; HW/HL = 1.38–1.54; basal margins almost asetose; dorsal punctation dense and coarse; eyes large (EYL/HL = 0.50–0.67), dorsolateral in large males, more lateral in females and smaller males, prominent and moderately protruding, lateral margins of head obscured by eye in dorsal view; HL1/HL2 not distinctly greater in females than males (♂ = 1.45–3.67, ♀ = 3.00–4.00); antennae as in Figure 18B View Figure 18 , antennomeres 1–6 black, 7–11 greyishblack; apex of antennomere 11 moderately to deeply emarginate medially; each pair of apical setae widely separated by apical emargination, one on each side of apex; mandibles as in Figure 18A View Figure 18 , longer than head in large males, shorter or subequal to head in females (ML/HL ♂ = 0.87–1.30, ♀ = 0.81–1.00), T1 larger than T2 and T3. Thorax and abdomen. Pronotum ( Fig. 18F View Figure 18 ) slightly transverse (PW/PL = 1.07–1.24), moderately to strongly narrowed posteriorly; PL 1.24– 1.44 ¥ ESL; basolateral margins faintly incised, hind angles indistinct; basolateral denticle of large males indistinct; with irregularly scattered peripheral setae and sparse short setae on lower anterolateral declivities; basolateral impressions asetose, asperate; scutellum imbricate with black vestiture; elytra uniformly black, rugosely sculptured, sparsely covered with very fine uniformly black vestiture ( Fig. 1H); glabrous humeral region large, impunctate; wings fully developed, black, but without distinct black spot in medial field between MP3 and MP4 veins; ventral pterothoracic vestiture golden brown, vestiture of legs black; abdomen with dull reflection due to characteristically imbricate microsculpture ( Fig. 19A View Figure 19 ), with short appressed and moderately dense vestiture; parasclerites of abdominal segment II completely fused (without apical notch); dorsal abdominal vestiture mostly black, with golden brown maculation mostly on tergites IV–V ( Fig. 1H); ventral abdominal vestiture golden brown on sternites III–VIII, with more black on VII and VIII; tergite VII with well-developed palisade fringe. Male genitalia. Aedeagus as in Figure 18E View Figure 18 ; median lobe apex short, narrowly subacute ( Fig. 18C View Figure 18 ); paired apicolateral sclerites (as) articulated to internal edge of median orifice, not fused to it. Paramere as in Figure 18I View Figure 18 . Internal sac inverted as in Figure 18E View Figure 18 , everted as in Figure 18H View Figure 18 ; ventral process (vp) with ventromedian spiculose strip (vr)> 2¥ length of ventral sclerite (vs); membranous sheath of copulatory piece (cp) reduced, with most of apical portion of cp exposed; appendix flared laterally ( Fig. 4D View Figure 4 , app). Female internal genitalia. Female genitalia as in Figure 18G View Figure 18 ; vaginal plate (vp) broadly bilobed posteriorly, with median sclerotized strip (ss); vaginal fold completely membranous, bursa copulatrix (bcx) distinctly longer than vaginal plate; spermatheca (sp) sausage-like, connected to bcx via short duct. Chaetotaxy. Basiantennal and basolateral pronotal macrosetae absent or vestigial, rarely present as pair; basisternal pair absent; ps1 absent, humeral macroseta usually absent, if present then rarely on both elytra; elytral discal series with 3–4 macrosetae; posterior elytral patch variable, basohumeral macrosetae usually absent, if present only one, and rarely on both elytra, posterior epipleural macroseta present or absent; tergal chaetotaxic formula = 2-2-2-2-2-4 (unique in Creophilus ), medial macrosetae absent on tergites III–VII; inner lateral macrosetae absent on tergites III–VIII; second gonocoxal macroseta usually absent, if present rarely on both gonocoxites.
Variation: The extent of abdominal vestiture patterning varies little, with tergites IV and V consistently with densest golden brown vestiture and other tergites with either sparse golden brown patches or entirely black vestiture. Chaetotaxy of head, pronotum, and elytra is unusually variable.
Comparison: Creophilus galapagensis superficially resembles C. maxillosus villosus and C. incanus due to golden-brown abdominal vestiture concentrated on tergites IV and V, and golden-brown pterothoracic vestiture. The entirely black elytral vestiture, imbricate abdominal microsculpture ( Fig. 19A View Figure 19 ), and reduced abdominal chaetotaxy (all unique characters within the C. maxillosus -group) will consistently distinguish C. galapagensis from these two species. Creophilus galapagensis is distinctly smaller than its congeners; males attain a maximum size only twothirds that of C. maxillosus males.
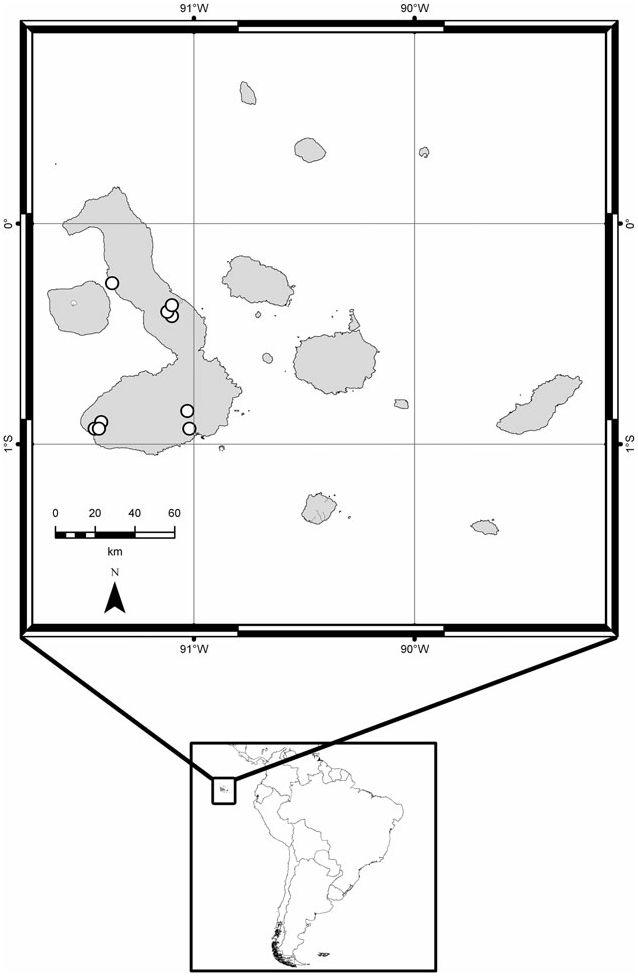
Distribution ( Fig. 20 View Figure 20 ): Galápagos Islands: Isla Isabela; Fernandina and Santiago ( Newton & Peck, 2006, as C. maxillosus , see below); San Cristóbal (Darwin’s specimens, see below).
Biology and ecology: Most collection records are from carrion traps. Other records are from under a dead bird ( Waterhouse, 1845) and from a decaying duck ( Franz, 1985). Habitat: agricultural areas, mixed forest, shrub forest, open forest, and pampas grassland. Altitude: 325–1100 m. Phenology: March– November. Newton & Peck (2006) listed it (as C. maxillosus ) as a predator. Other biology and lifehistory characteristics are unknown. Pupae and larvae are unknown.
Etymology: The species epithet ‘ galapagensis ’ is derived from a combination of Galapagos and the Latin adjectival suffix - ensis, meaning ‘belonging to’, in reference to the species being endemic to the Galápagos Islands.
Remarks: Known literature citations for Creophilus from the Galápagos Islands are most likely all C. galapagensis , recorded as C. maxillosus or C. villosus . The first records are three specimens (not examined by me) collected by Charles Darwin on Chatham Island (= San Cristóbal) during his famous ‘Beagle’ Expedition, and first reported as ‘ Creophilus , nov. spec.?’ with a description matching mine ( Waterhouse, 1845). Waterhouse (1877), Van Dyke (1953), and Franz (1985) listed it as C. villosus . Newton & Peck (2006) listed it as C. maxillosus , noting it may be a new species (on advice of A. Smetana). Waterhouse (1845) and Mutchler (1925) compared Darwin’s specimens to C. maxillosus and C. villosus . Linell (1898) listed it as ‘ Creophilus species’, noting it is probably C. villosus introduced from North America. Van Dyke (1953: 38) referred to three of the CAS paratypes listed above. Franz (1985) recorded nine specimens (not examined here) from the same localities on Isla Isabela as some paratypes.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.