Benedenia acanthopagr
|
publication ID |
https://doi.org/ 10.1080/00222930152023090 |
|
persistent identifier |
https://treatment.plazi.org/id/31398783-FFC6-703E-FE93-AA32A1DEFB9E |
|
treatment provided by |
Felipe |
|
scientific name |
Benedenia acanthopagr |
| status |
|
Benedenia acanthopagr i ( Hussey, 1986) comb. nov.
(®gures 9A±D, 10A, 12E)
Synonym. Tareenia acanthopagr i Hussey, 1986 (new synonym).
Material studied. NHML: No. 1984.9.13 .1 (holotype and paratype) (1 slide, 2 specimens: holotype labelled 1984.9.13.1; another specimen on same slide labelled paratype); NHML: No. 1984.9.13.2-11 1 (paratypes) (5 slides including 2 slides of sections, 3 slides each with 1 whole-mount) ex skin of Acanthopagrus latus (Houttuyn) (Sparidae) from ®sh in culture tanks at Al-Raas, Kuwait.
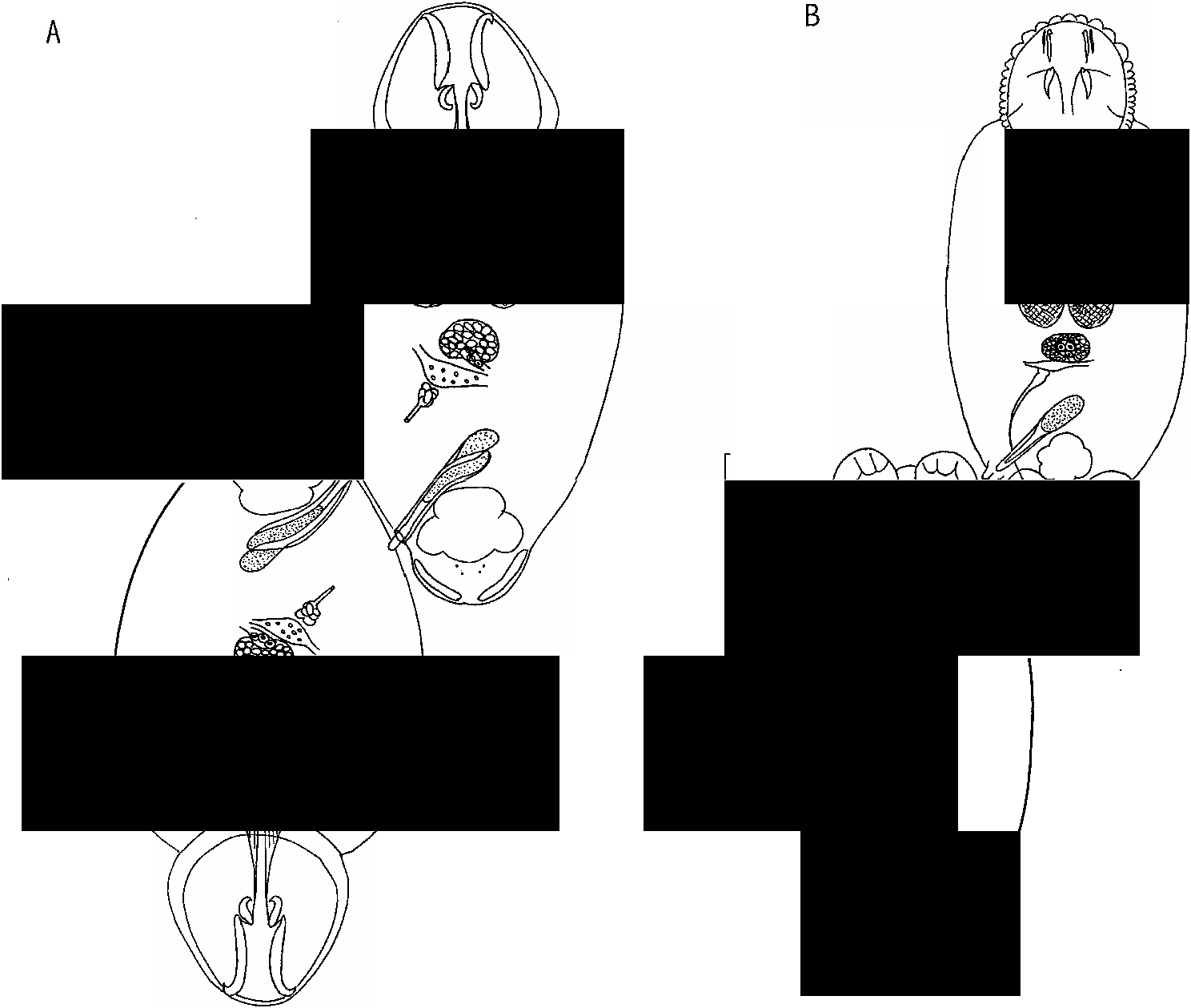
Observations. The original description matches the type specimens of Tareenia acanthopagri and Hussey (1986) should be consulted for a detailed account. We wish to point out a few important details about the anatomy of this worm. The marginal valve has a characteristic arrangement of lobes which was represented faithfully by Hussey (1986; his ®gure 1) but not described. From our study of type material, the arrangement is shown in ®gure 9A and can be described as: one lobe between hooklets of pair II on the posterior border of haptor; one lobe between each hooklet of pair II and position of hamuli tips; one lobe between position of hamuli tips and each of hooklet pair III; one lobe between hooklets III and IV, between IV and V, and between V and VI; up to nine small lobes (appearing fused) between hooklets VI and VII; approximately 10 small lobes (appearing fused) between hooklets VII and VIII; numbers of lobes between hooklets of pair VIII di cult to determine (details obscured by body proper) but Hussey (1986; ®gure 1) shows nine small (fused) lobes, which are visible in a scanning electron micrograph in his ®gure 7.
Other features of the haptor deserve comment. It has an unusual shape described by Hussey as resembling an`inverted tulip ¯ower’. The anterior hamuli are extremely long and span almost half the total length of the haptor (®gure 9A). The anterior hamuli just overlap the proximal ends of the accessory sclerites and, in some specimens, overlap totally the posterior hamuli (®gure 9A) whereas in other specimens, the posterior hamuli extend beyond the distal tips of the anterior hamuli.
Hussey (1986) erected Tareenia on the basis of two features: the vaginal pore opening anterior and ventral to the common genital pore and the presence of a ventral ¯ap which overlies the common genital pore (®gure 10A). The ventral ¯ap is clearly visible in type material but the anterior route of the vagina and its pore is very di cult to see in the types. Much of the rest of this species appears to be typically Benedenia -like. Hussey (1986) commented that he could not determine, even in sectioned material, whether the accessory gland reservoir was located inside the penis sac. Our investigation has shown that the wall of the penis canal is weakly muscular as is the accessory gland reservoir ( Hussey, 1986; ®gure 8) which lies proximal to the penis and is enclosed by a proximal extension of the wall of the penis canal. Hussey (1986) described, and we con®rm, that glands of Goto are present in the posterior angle between the testes.
Type-host and locality. Acanthopagrus latus (Sparidae) , in culture tanks, Al-Raas, Kuwait.
Published record and description. Hussey (1986).
Published host record. Sparidae : Acanthopagrus latus .
Site . Skin.
Distribution. From ®sh held in culture at Al-Raas, Kuwait.
Remarks. With the evidence available to him at the time, we consider Hussey’s judgement to establish a new genus, Tareenia , based on two unique characters, (1) vagina opening anterior and ventral to common genital pore and (2) ventral ¯ap near the submarginal common genital pore (®gure 10A), to be reasonable. However the progressive revision of some benedeniine genera, the clari®cation of some generic characters and recent descriptions of two species also with an anterior vagina, but placed in Benedenia , calls for a reappraisal of Tareenia .
Tareenia acanthopagr i from the skin of Acanthopagrus latus (Sparidae) View in CoL from Kuwait was the ®rst benedeniine described to possess a vagina opening anterior to the common genital pore (®gures 10A, 12E). Subsequently, however, two benedeniine species with an`anterior vagina’ have been described and placed in Benedenia View in CoL . Byrnes (1986) described B. anticavaginata View in CoL from`under pectoral ®ns’ of Acanthopagrus australis (Owen) View in CoL and A. berda (ForsskaÊl) (Sparidae) View in CoL in Australia, with a vagina opening anterior to the common genital pore on the dorsal surface (®gure 10B). Whittington and Kearn (1993) reported B. lutjani View in CoL with a vagina also opening anterior to the common genital pore on the dorsal surface of the parasite (®gures 10C, 12F) from all ®ns (predominantly the pelvics) but also from the body ¯anks and head of Lutjanus carponotatus (Richardson) (Lutjanidae) View in CoL in Australia. At least one other Benedenia species , as yet undescribed, with a vagina opening anterior and dorsal to the common genital pore, is known to occur on the gills of Diagramma labiosum (Macleay) (Haemulidae) View in CoL in Australia (Whittington and Deveney, previously unpublished observation). Recently, Egorova (1997) placed B. lutjani View in CoL and B. anticavaginat a in Tareenia View in CoL , but she did not resolve fully the similarities and diOEerences between these species. Hussey (1986) agreed his new species ®tted the concept of Benedenia View in CoL as interpreted by several authors but compared it directly with the type species, B. sciaenae View in CoL . Although not stating so, Hussey appeared to base his comparisons of T. acanthopagr i on redescriptions of B. sciaenae View in CoL by Goto (1899) and Palombi (1949) and considered, therefore, that B. sciaenae View in CoL possessed a common genital pore which united with the vaginal pore to form a common genital atrium. Hussey commented that B. sciaenae View in CoL diOEered, therefore, from most other species later allocated to Benedenia View in CoL and suggested the desirability of restricting Benedenia View in CoL to species with this character. We demonstrated above, however, that B. sciaenae View in CoL is as originally described by van Beneden (1856, 1858), possesses separate male, female and vaginal pores and have revised the generic diagnosis to accommodate this. It was further argued by Hussey (1986) that the anterior position of the vaginal pore in T. acanthopagr i may be an important taxonomic character reēcting the reproductive behaviour and method of sperm exchange in this species. Egorova (1997) based her opinion of the generic composition of Tareenia View in CoL largely on the anterior position of the vagina. We argue, however, that an anterior shift in the position of the vaginal opening seems unlikely to have large repercussions for the way the parasites approach each other during mating. Figure 11 View FIG shows diagrammatically that there is likely to be little diOEerence in the way species with a vagina located anterior to the common genital pore mate (®gure 11B) in comparison with species possessing a vagina posterior to the common genital pore (®gure 11A). Furthermore, Kearn and Whittington (1992) demonstrated that diOEerent species of Benedenia View in CoL can cross-inseminate in at least two diOEerent ways: by spermatophore s or by intromission without spermatophores. Thus, there is a need to determine more about the speci®c method(s) of copulation in species with an anteriorly located vaginal pore before schemes proposing diOEerent reproductive behaviours and reproductive isolation are propounde d further.
On present evidence, we consider that the position of the vaginal pore is a speci®c variable that has probably evolved more than once in this group of monogeneans. Other diOEerences in the position of the vagina occur elsewhere in species of Benedenia View in CoL (following our conception of the genus) and ®gure 12 shows the continuum of variation we have observed. The vagina of B. sciaenae View in CoL opens close, but just posterior, to the separate male and female pores (®gures 4, 6A) and a similar position has been noted of the vagina relative to the common genital pore in B. monticellii View in CoL (®gure 12D) whereas in B. ovata Goto, 1894 View in CoL , the vagina opens far more medioposteriorly (®gure 12A); there are other, intermediate conditions for the position of the vaginal pore depicted in ®gure 12B, C. Presently, we consider the best course of action is to include all species with diOEerent vaginal pore positions but otherwise of similar morphology in Benedenia View in CoL pending further investigations. It is possible that the continuum of speci®c variation in the position of the vagina may become more evident as more species are described, thus making the diOEerentiation between species with an`anterior’,`median’ or`posterior’ vagina redundant. Furthermore, within species described with an`anterior’ vagina, there is variation in the location of the vaginal pore: in B. acanthopagr i, the pore is ventral whereas in B. anticavaginata View in CoL and B. lutjani View in CoL , the pore is dorsal. Thus our decision is that Tareenia Hussey, 1986 View in CoL be synonymized with Benedenia View in CoL and the three species, T. acanthopagr i, T. anticavaginata View in CoL and T. lutjani View in CoL of Egorova (1997) become Benedenia acanthopagr i comb. nov., B. anticavaginata View in CoL and B. lutjani View in CoL , respectively. If future studies should show extra and signi®cant uniting characters between these species other than the anterior position of the vaginal pore, their generic status should be reappraised further.
The ventral ¯ap close to the ventral common genital pore was another important character in Hussey’s decision to erect Tareenia . The report of prominent lobes associated with the common genital pore in B. lutjani by Whittington and Kearn (1993) (®gure 10C) and the discovery during the present study of a lobe associated with the common genital pore of B. anticavaginata (®gure 10B and p. 685), may appear at ®rst, to support the validity of Tareenia , but the presence of lobes and ¯aps has been noted in other species of Benedenia which do not possess an`anterior vagina’, such as B. epinepheli as redescribed by Ogawa et al. (1995) and B. hawaiiensis as reappraised in this study. The number, shape and position of these lobes and ¯aps diOEer between species, their role is unknown, and it remains to be determined whether the diOEerent protuberances have similar functions.
Benedenia acanthopagr i can be distinguished from other Benedenia species that possess an`anterior vagina’ by: the position of its vaginal pore which is ventral; the presence of a ventral ¯ap from the body which covers the common genital pore (®gure 10A); the unusual shape of the haptor (®gure 9A); the presence of very long, slender and straight anterior hamuli which span almost half the total length of the haptor, which overlap the accessory sclerites proximally, and which entirely or almost entirely overlap the posterior hamuli (®gure 9A).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Benedenia acanthopagr
| Whittington, I. D., Deveney, M. R. & Wyborn, S. J. 2001 |
B. lutjani
| Whittington and Kearn 1993 |
B. lutjani
| Whittington and Kearn 1993 |
B. lutjani
| Whittington and Kearn 1993 |
T. lutjani
| Whittington and Kearn 1993 |
B. lutjani
| Whittington and Kearn 1993 |
B. anticavaginata
| Byrnes 1986 |
Tareenia
| Hussey 1986 |
Tareenia
| Hussey 1986 |
B. anticavaginata
| Byrnes 1986 |
Tareenia
| Hussey 1986 |
T. anticavaginata
| Byrnes 1986 |
B. anticavaginata
| Byrnes 1986 |
B. monticellii
| Johnston 1929 |
B. sciaenae
| Odhner 1905 |
B. sciaenae
| Odhner 1905 |
B. sciaenae
| Odhner 1905 |
B. sciaenae
| Odhner 1905 |
B. sciaenae
| Odhner 1905 |
B. sciaenae
| Odhner 1905 |
B. ovata
| Goto 1894 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |
Benedenia
| Diesing 1858 |