Aquilegia cremnophila Bacch., Brullo, Congiu, Fenu, J. Garrido & Mattana, 2012
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.56.1.9 |
|
persistent identifier |
https://treatment.plazi.org/id/03DE87C8-FFD0-FF83-5AE6-994B73CFFD6B |
|
treatment provided by |
Felipe |
|
scientific name |
Aquilegia cremnophila Bacch., Brullo, Congiu, Fenu, J. Garrido & Mattana |
| status |
sp. nov. |
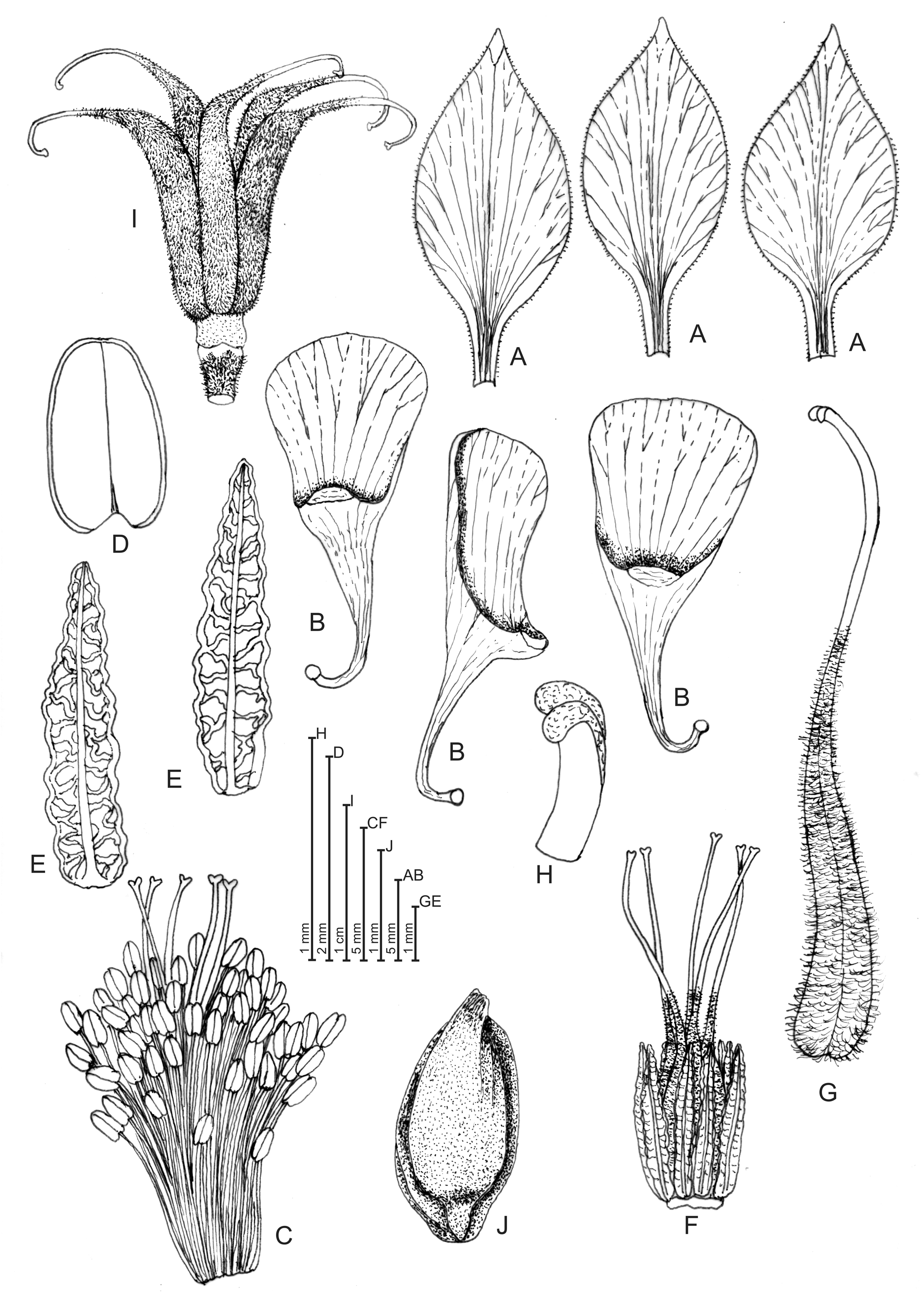
Aquilegia cremnophila Bacch., Brullo, Congiu, Fenu, J. Garrido & Mattana View in CoL , sp. nov. ( Fig. 1–2 View FIGURE 1 View FIGURE 2 )
Aquilegia nugorense affinis sed caulibus minoribus, periantio azureo-violaceo, lamina petalis majore, calcare arcuatouncinato differt.
Type:— ITALY. Sardinia: Palumbrosa ( Monte Corrasi , Oliena - Nuoro ), calcari mesozoici, pareti rocciose ombreggiate, 40° 14' N – 09° 25' E, 1330 m, 5 June 2011, G GoogleMaps . Bacchetta , G . Fenu & E . Mattana , s.n. (holotype CAT!, isotypes CAG!, CAT!, FI!) .
Perennial herb with rhizome branched at ground level in 2–3 caudicles. Stems 2–3, erect, rigid, (18–) 24–30 cm tall, pubescent with patent hairs 0.3–0.5 mm long, 1–2(–3) branched above. Basal leaves in rosette, more or less numerous; petiole (6.0–)9.5–11.5(–13.0) cm long, covered with patent pubescent and woolly hairs 0.3– 0.5 mm long, 2-ternate (rarely 1-ternate), with primary petioles 1.5–6.0 cm long and secondary ones 0.2–1.5 cm long; leaflets tripartite, 1.5–3.0 × 1.5–3.5 cm, green-greyish and glabrescent beneath, green and pubescent above, with appressed hairs 0.1–0.2 mm long, irregularly cuneate-obovate, more or less incise-lobate, with lobes obtuse to subacute, mucronate. Cauline leaves 1–2 like basal ones; petiole (1.0–)3.5–5.5(–7.5) cm long, the uppermost bracteate, ternate or simple, shortly petiolate or sessile. Pedicels densely covered by glandular hairs. Flowers 4–10, suberect or reclinate in bud, suberect or erect at anthesis, (40.5–)48.5–54.5(–59.0) mm in diameter. Sepals blue-violet (rarely lilac), patent, (18–)20–26(–29) × (7.5–)9.5–11.5(–12.5) mm, multinerved, ovate-lanceolate, glandular-puberulous outside, glabrous inside; apex acute, yellow-greenish; keel greenish. Petals concolourous, sometime lilac inside, suberect, (21.0–)25.5–31.5(–33.5) mm long (spur included), multinerved; blade glabrous, truncate-rounded or rounded at apex, (10.0–)12.0–16.5(–19.0) × (8.5–)10.5– 13.0(–13.5) mm. Spur funnel-shaped, curved to curved-uncinate at apex, 14.0–17.5(–19.0) mm long, rigid, glabrous or subglabrous with scattered patent hairs; nectary yellow-greenish. Stamens numerous, unequal, with filament hyaline, (8.5–)9.0–12.0(–13.5) mm long. Staminodes 10, hyaline, linear-lanceolate, (5.8–)6.1– 8.4(–11.8) × (0.8–)1.0–1.2(–1.4) mm, keeled, strongly wrinkled, acute and revolute at apex. Anthers lemonyellow, 1.6–2.6 × 1.1–1.5 mm. Pistils 12–15 mm long; ovary densely hairy-glandular; style (6.5–)7.3–9.9(– 10.9) mm long, erect-curved, hairy below, persisting in fruit; stigma grooved dorsally, curved and bilobed at apex. Carpels (4–)5–6(–8), densely glandular hairy. Fructiferous peduncles erect or suberect. Immature follicles green, erect, (18–)23–28(–30) × 4–5 mm, divaricate-patent above and curved at apex, glandularpubescent, markedly reticulate-veined. Mature follicles pale-brown, (17.0–)21.5–25.5(–30.0) × 3.0– 4.5 mm. Seeds trigonous or ovoid-trigonous, ribbed, black and shining, 2.22–2.62 × 1.23–1.49 mm.
Habitat:— Aquilegia cremnophila is a chasmophyte growing on Mesozoic dolomitic limestones, at an elevation of 1300–1420 m. It likes the rocky walls, colonizing the shady crevices. The species is a member of a rupestrian plant community rich in Sardinian and Cyrno-Sardinian endemics, such as Armeria morisii Boiss. , Campanula forsythii (Arcangeli) Podlech , Euphorbia amygdaloides subsp. semiperfoliata (Viv.) Radcl. -Sm., Hieracium supramontanum Arrigoni.
Distribution:—This species is circumscribed to the upper part of Mt. Corrasi, municipality of Oliena (Nuoro - Sardinia), where it is represented by only four nuclei ( Garrido et al. 2012).
Etymology:—From the Greek words “ cremnos ” = cliff, and “ philos ”= fond of, in reference to its habitat.
Phenology:—Flowering late May to June, fruiting July to August ( Mattana et al. 2012).
Conservation:—Although the four nuclei are threatened by goat and mouflon grazing, a population decline was not observed. However, considering the small size population and the possibility that the level of threat could quickly increase (i.e. human activity or stochastic events), Aquilegia cremnophila we applied the IUCN criteria (2001) and propose an IUCN red list category as vulnerable: VU = D2, as the total number of mature plants ranged from 250 to 1000, distributed in less than 10 km 2.
As part of the projects funded by the Regione Autonoma della Sardegna, the Centro Conservazione Biodiversità (Università degli Studi di Cagliari) initiated a conservation programme of in situ studies and long-term ex situ conservation at the Sardinian Germplasm Bank ( BG-SAR) for all Aquilegia species endemic of Sardinia, including A. cremnophila .
Observations:— Aquilegia cremnophila represents an endemism circumscribed to the Mt. Corrasi (Central-Eastern Sardinia). It shows close relationships mainly with A. nugorensis and also with A. nuragica , species occurring in the same Sardinian territories. In particular A. cremnophila differs from A. nugorensis in having smaller size, perianth blue-violet, petal blade larger (12 – 16 × 10 – 13 mm), spur curved and uncinated at apex ( Fig. 3 View FIGURE 3 ). Besides, they are well differentiated from a phenological and ecological point of view, since A. cremnophila is an calcicolous orophyte having a later fruiting ( Mattana et al. 2012), exclusive of shady rupestrian habitat, behaving as a true chasmophyte. As concerns A. nugorensis , it is a nemoral hygrophyte growing along the streams in the canopy of riparian woods and is indifferent to the geological substratum ( Arrigoni & Nardi 1978). This new species differs also from A. nuragica for the stems and petioles pubescent, perianth colour and spur longer (14 – 20 mm), as well as for the ecology. In fact, A. nuragica grows on vertical rocks just next of petrifying springs with tufa formation.
The morphological and ecological differentiation of Aquilegia cremnophila is also supported by the molecular and the eco-physiological investigations carried out by Garrido et al. (2012) and Mattana et al. (2012), respectively. In particular, Garrido et al. (2012), studying the spatial genetic structure of the Aquilegia taxa occurring in Sardinia, found that the analyses of AFLP multilocus individual profiles were not fully compatible with the current taxonomic affiliations of Sardinian taxa. The population of Mt. Corrasi, now attributed to A. cremnophila , represents a spatial genetic group well differentiated from all the other species, including A. nugorensis . Data regarding the germination behaviour of Sardinian Aquilegia , put in evidence that the riparian species ( A. barbaricina and A. nugorensis ) germinate only after warm and cold stratification pre-treatments, while the rupestrian species ( A. cremnophila ) germinates also without any pre-treatments at warm temperatures ( Mattana et al. 2012), as detected for many others Mediterranean orophytic plants ( Giménez-Benavides et al. 2005).
Paratypes:— ITALY. Sardinia: Ahottadoglios (Monte Corrasi, Oliena - Nuoro), 1345 m, 5 June 2011, G . Bacchetta , A . Congiu , G . Fenu & E . Mattana, s.n. ( CAG); Arco Corrasi (Monte Corrasi, Oliena - Nuoro), 1370 m, 5 June 2011, G . Bacchetta , A . Congiu , G . Fenu & E . Mattana, s.n. ( CAG); Punta Corrasi (Monte Corrasi, Oliena - Nuoro), 1420 m, 5 June 2011, G . Bacchetta , A . Congiu , G . Fenu & E . Mattana , s.n. ( CAG) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |