Amygdalops sevciki, Roháček, 2018
|
publication ID |
https://doi.org/ 10.2478/aemnp-2018-0007 |
|
publication LSID |
lsid:zoobank.org:pub:9808C120-13B7-43F8-B735-C13D2B6D43CA |
|
DOI |
https://doi.org/10.5281/zenodo.3681341 |
|
persistent identifier |
https://treatment.plazi.org/id/03AAB202-7C7F-FFF0-FE99-FC08FCFC6506 |
|
treatment provided by |
Tatiana |
|
scientific name |
Amygdalops sevciki |
| status |
sp. nov. |
Amygdalops sevciki View in CoL sp. nov.
( Figs 45–61 View Figs 45–50 View Figs 51–55 View Figs 56–61 , 63 View Figs 62–67 )
Amygdalops bisinus View in CoL (misidentification): ROHÁČEK (2008: 341) View Cited Treatment (partim, females only).
Type material. HOLOTYPE:, labelled: ‘ CHINA: Hainan I.: Sanya 30 km NE, Sandaozhen env. Xinjiancun , 22.v.2016, 18°28ʹ44ʺN 109°38ʹ23ʺE, sweeping, J. Ševčík leg.’ and ‘ Holotypus, Amygdalops sevciki sp. n., J. Roháček det. 2017’ (red label). The specimen is dissected and (after being used for DNA extraction) preserved in glycerine in a pinned plastic microvial; its abdomen is detached and preserved with dissected genitalia in glycerine in a plastic tube pinned on the same pin ( SMOC). GoogleMaps
PARATYPES: 1 ♀, with same data, dissected, prepared, partly used for DNA extraction and preserved in glycerine as in the holotype including the abdomen ( SMOC). Three further paratypes (originally misidentified and designated as paratypes of Amygdalops bisinus by ROHÁČEK 2008: 341): GoogleMaps THAILAND: Mae Fang N. P., No. 14, over & along forest brook, 1.xi.2004, 1 ♀, L. Papp & M. Földvári leg. ( HNHM, genit. prep.). GoogleMaps INDONESIA: Isle Flores: 8,49 S, 121,02 E, eastern periphery of village Mataloko, ca 10 km ESE Badjawa, 200–300 m E mission church and school, X 859, creek valley, open cultivated land (vegetables, manioc, diverse herb. vegetation (2 m height), grazed by buffaloes, 24.ix.1992, 1 1 ♀, M. v. Tschirnhaus leg. ( ZSMC, both dried from ethanol, genit. prep.).All paratypes with same type label as the holotype but it is yellow and has ‘Paratypus’ instead of ‘Holotypus’. GoogleMaps
Description. Male. Total body length 1.86–1.99 mm. Body bicolourous, brown and yellow. Head slightly longer than high. Occiput concave, entirely blackish brown, subshining, with a pair of large greyish microtomentose spots laterodorsal to foramen. Frontal triangle very long and narrow, almost reaching to anterior margin of frons, with very acute anterior corner, largely bare and shiny, including ocellar triangle. Frons almost entirely dark, paler brown only in anterior fifth or fourth; stripes delimiting frontal triangle from orbits somewhat depressed, darker, greyish microtomentose and dull. Orbit largely dark brown and shining, with anterior fourth somewhat paler and only its foremost part lateral to base of antenna whitish yellow and dull. Face dark yellow to (ventrally) ochreous; parafacialia and gena almost white, with silvery white microtomentum but both pale-brown to ochreous margined (very narrowly on gena); postgena with only ventral corner whitish yellow, otherwise concolourous with dark brown occiput. Mouthparts dirty yellow including clypeus and palpus. Cephalic chaetotaxy (partly reconstructed from paratypes): pvt small but distinct and crossed; vti relatively short, only about 1.5 times as long as but thicker than pvt; vte (together with posterior ors) longest of cephalic setae; oc relatively short but somewhat longer than vti; 2 long ors, posterior as long as vte, anterior only slightly shorter; 2 microsetulae in front of anterior ors, anterior microsetula only slightly shorter than posterior; 2 pairs of microsetulae at sides of anterior thirds to fourth of frontal triangle; 1 relatively weak vi and 1 subvibrissa about three-fourths of vi; peristomal setulae sparse, only 4–5, being somewhat longer anteriorly, shorter posteriorly; postocular setulae very minute; palpus with usual subapical seta. Eye large, covering most of head profile, very convex, with longest diameter about 1.5 times as long as shortest one. Gena anteriorly very narrow; its shortest height 0.05 times as long as shortest eye diameter. Antenna strongly geniculate between pedicel and 1st flagellomere, with yellowish white scape and pedicel but the latter narrowly orange ochreous at anterior margin; 1st flagellomere dirty yellowish white but distinctly brown darkened in dorsal half, darkest around base of arista, whitish long ciliate on apex. Arista blackish brown, about 1.9 times as long as antenna, long-pectinate.
Thorax distinctly narrower than head, bicolourous, dark brown and pale yellow. Mesonotum including scutellum brown to dark brown but somewhat lighter than head. Humeral and notopleural areas ochreous yellow; pleural part of thorax with broad (covering entire propleuron and most of mesopleuron) dark brown dorsal band extending from propleuron to haltere and sharply delimited from whitish yellow ventral portion of pleura. Thoracic chaetotaxy: 1 relatively short hu, 2 npl (anterior stronger than hu), 1 very small and fine prs; only 1 (posterior) long dc about as long as apical sc and situated close to scutellum (anterior dc reduced to microseta); ac microsetae sparse, in 4 rows on suture, in 2 rows between posterior dc; sa shorter than pa, both relatively short; 2 sc, apical long and strong (together with posterior dc longest thoracic setae), laterobasal sc about half length of apical sc; ppl reduced, not visible; 2 stpl, posterior longer and thicker; only 3 additional setae on ventral part of sternopleuron. Scutellum rounded triangular with slightly convex dorsal surface; postscutellum well developed, convex, blackish brown. Legs bicolourous, largely yellow to pale yellow, with femora diffusely brownish darkened in distal fourth to third (except for yellow knees) and with tibiae similarly but less darkened in proximal fourth; these brownish markings less distinct on f 1 and t 1. Pedal chaetotaxy: f 1 with usual posterodorsal and posteroventral row of longer setae, those posteroventral in distal half longest; f 2 without peculiarities and t 2 with usual ventroapical seta; f 3 with posteroventral row of setae along entire length but only 5 of them in distal fifth to third shortened, thickened and in denser comb ( Fig. 51 View Figs 51–55 ). Wing ( Fig. 63 View Figs 62–67 ) with simplified pattern, thus with only preapical spot darker brownish (but paler than in most relatives), rest of wing membrane almost unicolourous, with area between R 4+5 and C only a little lighter. R 4+5 and M subparallel, with very slight preapical convergence; r-m situated near middle (slightly in front of midpoint of) dm cell. Anal lobe and alula reduced. Wing measurements: length 1.78–2.02 mm, width 0.53–0.58 mm, Cs 3: Cs 4 = 1.68–1.82, r-m\dm-cu: dm-cu = 3.54–4.00. Haltere with pale brown stem and dark brown knob.
Abdomen. Preabdominal terga large, with short and relatively thick setae, uniformly brown to dark brown, thus without paler laterobasal spots on T4 and/or T5. T6 submembranous, short, bare and very pale. Preabdominal sterna relatively narrow, and finely setose, paler brown than terga, and becoming somewhat wider posteriorly, hence S5 the largest. S6–S8 dark brown, dorsolaterally fused; S6 ventrally shortened, transversely band-like; S7 almost twice as long as than S6, somewhat rectangular, with ventral side shorter than dorsal; both S6 and S7 with anterior darkened marginal ledge-like stripe (thicker in S6) and each with 2 or 3 small setae; S8 relatively long, with thicker setae (as in T5) in posterior half.
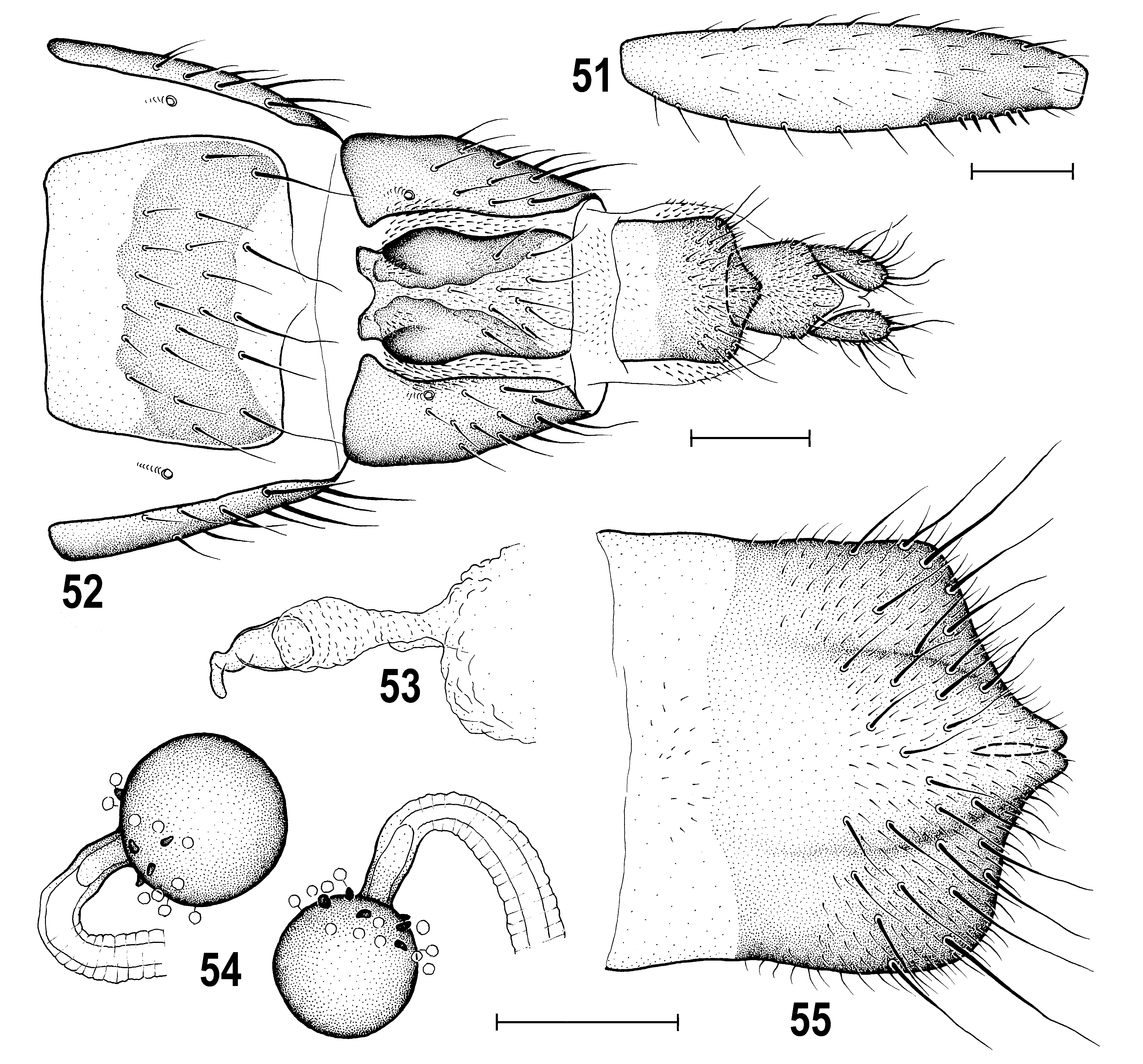
Genitalia. Epandrium hemispherical, medium-long ( Figs 49, 50 View Figs 45–50 ), rather sparsely setose, with 1 dorsomedial and/or 1 dorsolateral pair of longer setae; anal opening relatively small, narrowly subpentagonal ( Fig. 50 View Figs 45–50 ). Cercus small, distinctly shorter than gonostylus, finely setose. Medandrium ( Figs 49, 50 View Figs 45–50 ) comparatively high and narrow, ventrally narrower than that of A. bisinus . Gonostylus ( Fig. 48–50 View Figs 45–50 ) relatively small (much shorter than epandrial height), sinuous in profile and hence somewhat resembling that of A. bisinus but differing from the latter in having distal half more robust in lateral view ( Fig. 48 View Figs 45–50 ) and more tapered in caudal view ( Fig. 50 View Figs 45–50 ); also micropubescent pattern of outer side of gonostylus similar to that of A. bisinus including bare anterior margin and apex; setosity of inner side of gonostylus also similar in both species. Hypandrial complex ( Fig. 45 View Figs 45–50 ) markedly different from that of A. bisinus : hypandrium slender (also anteriorly), with reduced (membranous) internal lobes; transandrium ( Fig. 47 View Figs 45–50 ) simple, relatively slender laterally (not projecting ventrolaterally – cf. Fig. 45 View Figs 45–50 ), more narrowly concave ventromedially ( Fig. 47 View Figs 45–50 ); caudal process small, narrow, inconspicuous and short, transient to spinulose basal membrane, thus somewhat resembling that of A. cuspidatus Roháček, 2008 (cf. ROHÁČEK 2008: 347, Fig. 41 View Figs 38–44 ) although medially undivided. Pregonite ( Fig. 45 View Figs 45–50 ), low, incurved, slightly posteroventrally projecting and shortly separated by narrow posterior incision, with about 5 setae along posterior half of its ventral side. Postgonite ( Fig. 45 View Figs 45–50 ) very slender, knife-shaped, apically slightly bent and acutely pointed, having a few sensilla on outer side and 1 seta near middle of anterior margin; basal sclerite of postgonite markedly different from that of A. bisinus (cf. Fig. 35 View Figs 32–37 ), slender and about as long as postgonite. Aedeagal part of folding apparatus ( Fig. 46 View Figs 45–50 ) not darkened dorsally, relatively short and its external side with rather small flat tubercles (resembling that of A. cuspidatus); connecting sclerite well-sclerotized, proximally slender, triangularly dilated in middle part but distally attenuated and with series of small tubercles. Basal membrane ( Figs 45, 47 View Figs 45–50 ) with dense short spines arranged narrowly dorsomedially but more widely ventrally (cf. Fig. 47 View Figs 45–50 ). Aedeagal complex ( Fig. 46 View Figs 45–50 ) of the A. cuspidatus type. Phallapodeme relatively slender, but with somewhat dilated and bifurcate base and its apex with well-developed lateral projections; also fulcrum relatively slender.Aedeagus with short frame-like phallophore closely resembling that of A. cuspidatus (cf. ROHÁČEK 2008: Fig. 42 View Figs 38–44 ) and large distiphallus. Saccus of distiphallus relatively voluminous, membranous except for very short basal part and provided with two groups of fine spines, one in basal half, the other on right side of apex of the membranous part (see Fig. 46 View Figs 45–50 ). Filum of distiphallus very slender, formed by 2 dark stripe-like twisted sclerites which are closely attached except for distal third and terminate in a rounded membranous apex. Ejacapodeme small, short, with slender finger-like projection.
Female. Similar to male unless mentioned otherwise. Total body length 2.22–2.50 mm. Face somewhat darker, ochreous to pale brown; also mouthparts darker, with clypeus brown and palpus ochreous-brown; 1st antennal flagellomere more extensively brownish. t 2 with ventroapical seta longer and thicker; f 3 posteroventrally simply finely setulose, lacking a group of shortened ventral setae. Wing measurements: length 2.22–2.42 mm, width 0.67–0.73 mm, Cs 3: Cs 4 = 1.75–2.15, r-m\dm-cu: dm-cu = 3.57–3.92. Abdomen with preabdominal terga shorter, more transverse and all uniformly dark brown. T3–T6 becoming narrower posteriorly. Preabdominal sterna smaller, narrower and paler than in male, pale ochreous-yellow, only lateral margins of S3–S5 may be narrowly darkened; S3 and S4 subequal (or S3 slightly narrower) and distinctly narrower than S5; the latter as broad as S6 but longer.
Postabdomen ( Figs 52 View Figs 51–55 , 57–58 View Figs 56–61 ) moderately long. T6 large, markedly wider and slightly longer than T7, tapering posteriorly, with numerous dense, short and thick setae ( Fig. 57 View Figs 56–61 ), dark brown with pale anterior margin. S6 ( Figs 52 View Figs 51–55 , 58 View Figs 56–61 ) narrower than T7, pale ochreous both anteriorly and posteriorly and characteristically brown-darkened in the middle part, finely setose. T7 ( Fig. 57 View Figs 56–61 ) blackish brown, anteriorly shallowly emarginate and with anterolateral corners extended onto ventral aspect and embedding 7th spiracle (see Figs 52 View Figs 51–55 , 58 View Figs 56–61 ), densely setose like T6 but mainly in posterior half. S7 relatively small and narrow, abruptly tapered anteriorly ( Figs 52 View Figs 51–55 , 58 View Figs 56–61 ) and sometimes gradually narrowed posteriorly ( Fig. 58 View Figs 56–61 ), with characteristic pattern composed of light narrow medial area bordered by larger brown lateral parts being anteriorly blackish brown patterned; fine setae concentrated in front of posterior pale-pigmented part of S7. T8 dark brown and unusually narrow ( Fig. 57 View Figs 56–61 ), tapering posteriorly due to ventrally bent sides, with a few (1 longer) fine setae in posterior third. S8 ( Figs 52, 55 View Figs 51–55 , 58, 60 View Figs 56–61 ) relatively long and narrow, brown in posterior two-thirds to four-fifths and setose in posterior third to half, and with prominent posteromedial bulge ( Fig. 55 View Figs 51–55 ) having the usual mediodorsal incision very narrow. Internal sclerotization of genital chamber formed by 2 pairs of fused flat bent pale brown sclerites being widened anteriorly ( Fig. 59 View Figs 56–61 ); annular sclerite very thin and twisted several times; vaginal area finely spinulose (see Figs 59, 60 View Figs 56–61 ). Ventral receptacle vesiculate, submembranous, roughly bell-shaped, with smooth surface and digitiform, somewhat variably bent ( Figs 53 View Figs 51–55 , 56 View Figs 56–61 ) terminal projection. Spermathecae spherical ( Figs 54 View Figs 51–55 , 61 View Figs 56–61 ), relatively large (one distinctly larger than other) each with a few grain-like spines in basal part; duct cervix short to medium long. T10 ( Fig. 57 View Figs 56–61 ) small and narrow, about as long as wide, brown, with scattered microtomentum and 1 pair of longer posteromedial setae. S10 ( Figs 52 View Figs 51–55 , 58 View Figs 56–61 ) also small, slightly larger than T10, brown, micropubescent, posteromedially projecting, with fine setulae at posterior margin. Cerci ( Figs 52 View Figs 51–55 , 57 View Figs 56–61 ) medium-sized, brown, with moderately long fine setae.
Discussion. Although Amygdalops sevciki sp. nov. strikingly resembles A. bisinus Roháček, 2008 in the form of the gonostylus and some external characters (which led to previous confusion of these two species by ROHÁČEK 2008) these two species proved not to be closely related (see also their dissimilarity in COI, Fig. 31 View Fig ). This new species belongs to the A. cuspidatus subgroup of ROHÁČEK (2008). This can be demonstrated by a number of similarities in the construction of the male internal genitalia (cf. pregonite, basal sclerite of postgonite, caudal process of transandrium, armature of basal membrane and aedeagal part of folding apparatus, connecting sclerite) as well as in the female terminalia (cf. structure of S7, spherical spermathecae with short blunt spines). Amygdalops sevciki sp. nov. seems to be particularly related to A. cuspidatus Roháček, 2008 resembling the latter in most of the above male characters and to A. sp. cf. cuspidatus of ROHÁČEK (2008: Figs 52–57 View Figs 51–55 View Figs 56–61 ) from Taiwan in the shape of the female S7 and spermathecae. Actually, the latter (hitherto unnamed) species is possibly the closest relative of A. sevciki but this can only be demonstrated as and when its male is found and described. Female A. sp. cf. cuspidatus differs distinctly from that of A. sevciki sp. nov. in having yellow legs, broad and maculate S6 and wider, more transverse T6, T7, T8, T10, S8 and S10 (cf. ROHÁČEK 2008: Figs 53, 54 View Figs 51–55 ). Amygdalops sevciki can also be easily distinguished from A. cuspidatus by the sinuate lateral outline of the gonostylus, the more slender hypandrium and some detail in armature of the basal membrane and saccus of the distiphallus (for these structures in A. cuspidatus see ROHÁČEK 2008: Figs 38–43 View Figs 38–44 ), and (in female) also by the shape and pigmentation of T7, T8, S6, S7, S8, S10 and the more densely spinulose spermathecae (cf. ROHÁČEK 2008: Figs 45, 46, 48 View Figs 45–50 ).
Externally, A. sevciki is diagnosed by its very long and narrow frontal triangle, only 1 (posterior) dc seta, femora and tibiae with brownish annulus (in contrast to uniformly yellow legs in other species of the A. cuspidatus subgroup), male f 3 with a comb of only 5 short thickened posteroventral setae ( Fig. 51 View Figs 51–55 ), simplified wing pattern ( Fig. 63 View Figs 62–67 ) and both male and female preabdominal terga unicolourous brown, without paler lateral spots. Nevertheless, for its safe identification the examination of the male and female terminalia is recommended because the existence of further unnamed and similarly coloured species in the Oriental Region cannot be excluded.
Etymology. The species is named in honour of Jan Ševčík (Ostrava, Czech Republic), my friend, research colleague in dipterology and collector of this and other anthomyzids on Hainan Island.
Biology. The male holotype and female paratype of A. sevciki were netted by J. Ševčík on Hainan I. together with a pair of A. bisinus from lush vegetation near growth of longan ( Dimocarpus longan ) trees ( Fig. 65 View Figs 62–67 ). Other paratypes were found on herbaceous (sometimes partly grazed) vegetation beside streams in forested valleys. Adult occurrence was confirmed in May, September and November.
Distribution. The species is widespread in the Oriental Region: China (Hainan I.), Thailand and Indonesia ( Flores I.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Amygdalops sevciki
| Roháček, Jindřich 2018 |