Valdivianemertes valdiviae ( Bürger, 1909 )
|
publication ID |
https://doi.org/10.1080/00222933.2010.510251 |
|
persistent identifier |
https://treatment.plazi.org/id/AE6B87F8-7D44-EF1C-FE78-FD55E042FDC3 |
|
treatment provided by |
Felipe |
|
scientific name |
Valdivianemertes valdiviae ( Bürger, 1909 ) |
| status |
|
Valdivianemertes valdiviae ( Bürger, 1909) View in CoL
( Figures 1–42 View Figures 1–3 View Figures 4–6 View Figures 7 and 8 View Figures 9 and 10 View Figures 11–13 View Figures 14 and 15 View Figures 16 and 17 View Figures 18 and 19 View Figures 20–27 View Figures 28–34 View Figures 35–42 )
Figures 4–13 View Figures 4–6 View Figures 7 and 8 View Figures 9 and 10 View Figures 11–13 are taken from Bürger (1909: Taf, XXV, fig. 3; Taf. XXXI, figs. 1–9) via electronic digital imaging and have been relabelled in English. Figures 1–3 View Figures 1–3 presents maps, drawings and a photograph prepared by the authors. Figures 6–11 View Figures 4–6 View Figures 7 and 8 View Figures 9 and 10 View Figures 11–13 are photomicrographs taken by the authors.
Synonymy
Drepanophorus valdiviae Bürger 1909 ; Gering 1913; Brinkmann 1914 –15, 1917; Friedrich 1935, Stiasny-Wijnhoff 1936; Scharrar 1941.
Valdivianemertes valdiviae Stiasny-Wijnhoff 1923, 1936 View in CoL ; Kirsteuer 1973; Crandall 1993b, 1994, 2001.
Etymology
The generic name Valdivianemertes (gender feminine) Stiasny-Wijnhoff, 1923 is derived from the German research vessel Valdivia + NΗΜΕς τ´ Ης (the sea nymph Nemertes, daughter of Nereus and Doris). The name was not formed according to the strict rules of nomenclature in that it is a compound noun combining Greek and non-Greek elements. The specific epithet also commemorates the research vessel Valdivia .
Type specimens
The species was originally brie y described from a single specimen by Bürger some years after he had taken up his Professorship in the university at Santiago. A diligent search for his specimen at Santiago and at repositories in Europe to which the specimen might have been sent has not been successful. However, discovery of the current material, which agrees in all particulars with Bürger’s text and figures, now makes possible a full redescription of the species and designation of the current specimen as neotype. The specimen has been deposited in the Smithsonian Institution’s US National Museum of Natural History, as with all neotype material, under Bürger’s original name of Drepanophorus valdiviae as catalogue number 1126928.
Type locality
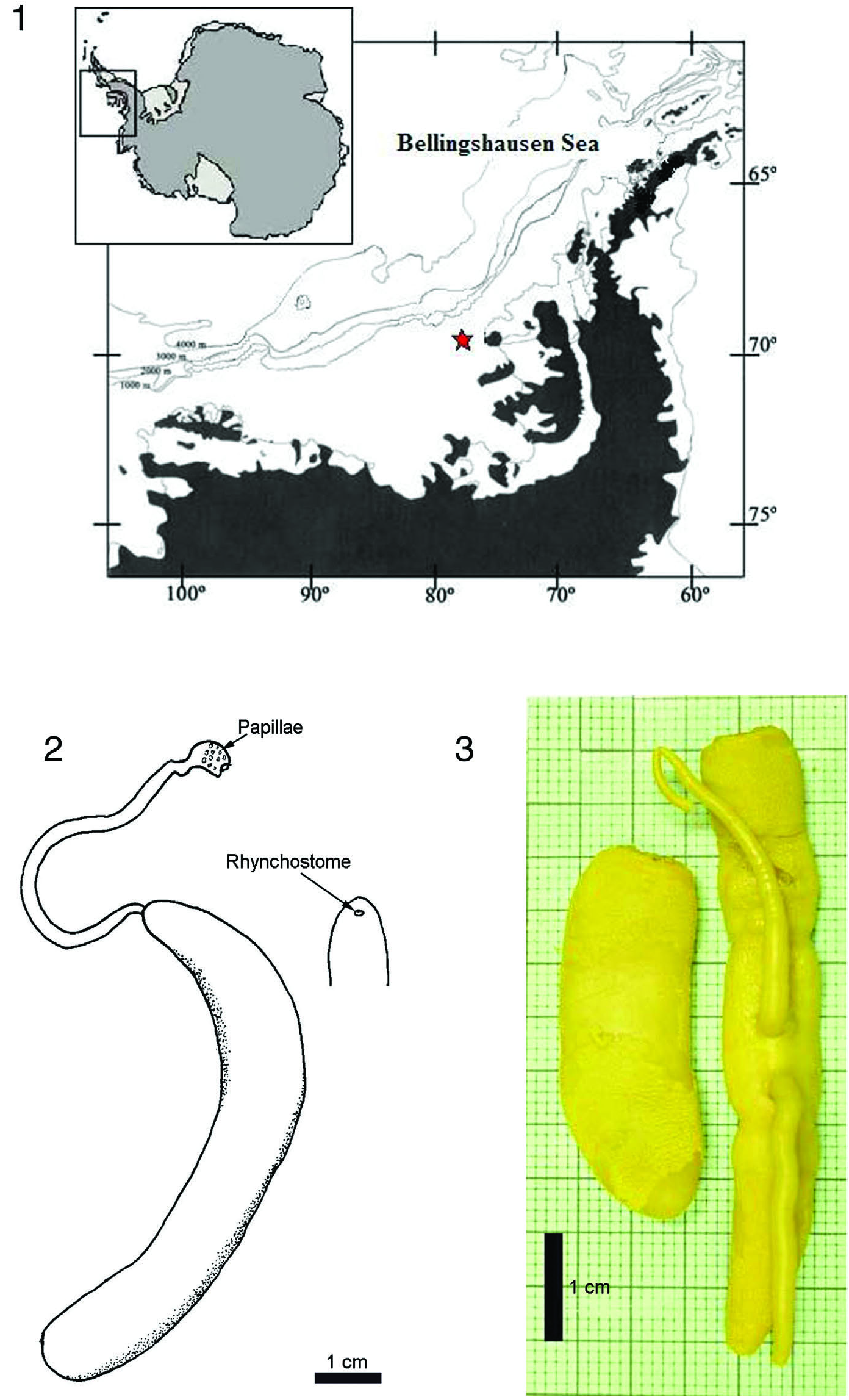
Bürger’s original description listed RV Valdivia Station 104. 35 ◦ 16.0 S, 22 ◦ 26.7 E, approximately 200 km east of southeast by east of Cape Agulhas, South Africa at a depth of 155 m. The neotype was collected by the RV Hespérides (BENTART-2003 cruise) at Station 13 in the Bellingshausen Sea, 69 ◦ 49 34 S, 77 ◦ 49 92 W, Antarctica, at a depth of 602 m ( Figure 1 View Figures 1–3 ) GoogleMaps .
Material examined
Bürger’s original specimen has not been located. The current specimen, herein designated as neotype, consists of 45 slides of the anterior body, 29 slides of the mid-body, 26 slides of the posterior body, and 14 slides of the detached proboscis.
Description
General. Bürger’s material consisted of a single specimen with complete body but missing proboscis, sketched from life by Braem, and notes accompanying the specimen. Bürger decided on quite limited grounds (cf. Systematic discussion) that taxon should be attributed to Drepanophorus but noted features which more closely resembled Amphiporus . Hence, his comparisons with various species of Drepanophorus (as then understood), in a number of passages quoted from his account, must be regarded with appropriate caution. Items from Bürger’s description are given in quotes first in each section (translation from the German by F.B. Crandall).
External features. “In life, judging from the figure ( Figure 4 View Figures 4–6 ), the specimen was 90 mm long and ranged from 6 to 9 mm wide. The preserved specimen was 55 mm long, ranged from 7 to 8 mm wide and was 4 mm thick. The body is attened, the head triangular and demarcated from the adjacent body by the prominent cephalic grooves. Also, the tail end was distinctly set off from the remainder of the body. This specimen outwardly resembles a Drepanophorus , particularly Drepanophorus crassus .”
Anaesthetized living neotype measured about 100 mm long and 10 mm in width. Body broad and attened, head demarcated from the body by anterior cephalic grooves. Body cross-section oval to triangular anteriorly, posteriorly dorsoventrally attened ending in a rounded spatulate shape ( Figure 2 View Figures 1–3 ). Everted proboscis about 2 mm in diameter, more than half the body length with the papillae very prominent at outer end ( Figures 2, 3 View Figures 1–3 ). Position of rhynchostome ventral somewhat behind tip of the head ( Figure 2 View Figures 1–3 ). No eyes visible.
Colour. Bürger notes that in life, the animal was yellow with medial portion a darker yellow but not sharply demarcated against light yellow to whitish edges ( Figure 4 View Figures 4–6 ). Cephalic grooves distinguished by dark yellow colour. Preserved specimen irregularly
grey in colour. Body of neotype specimen in life light cream with smooth, bright epithelium.
Ocelli. Eyes absent unlike all other genera of benthic Cratenemertidae .
Frontal organ. “A frontal organ equipped with a thick but very short frontal gland is present.” Frontal organ in its preserved, inverted position in neotype specimen is hemispheric depression at tip of head lined with usual pyramidal-shaped cells with clear cytoplasm and long agellar processes ( Figure 20 View Figures 20–27 ). Frontal gland about as wide as organ, extends only a short distance posteriorly.
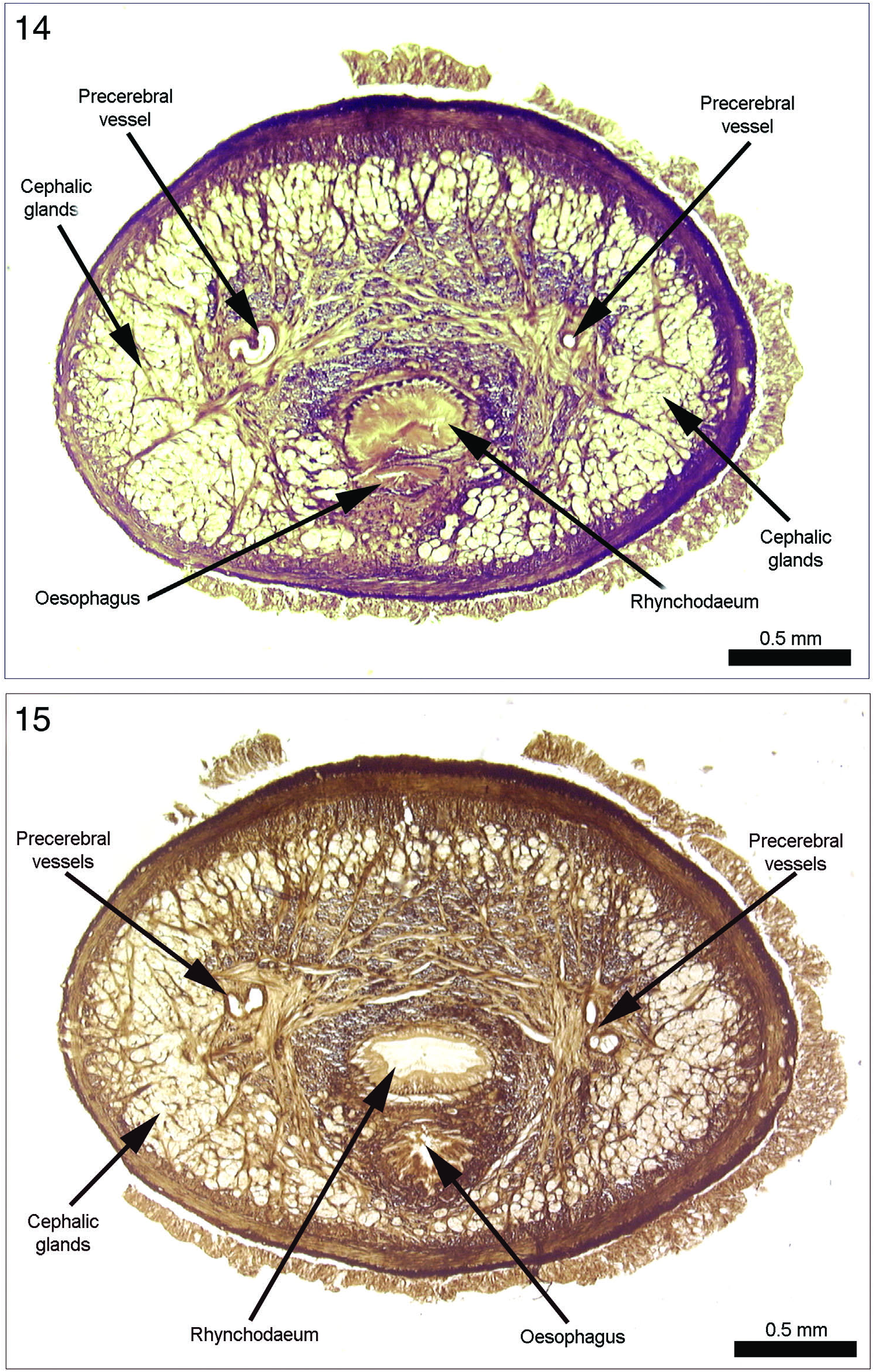
Cephalic glands. “These glands are developed all over in the outermost tip of the head, with the exception of the declivity where the proboscis and mouth opening are located. A little farther posterior they are present chie y in the sides of the head, but even here only up to the brain region ( Figure 5 View Figures 4–6 ).” Cephalic glands quite numerous, occupying entire circumference of anterior portion of head ( Figures 14 View Figures 14 and 15 , 15, 21, 23), emptying through body wall via individual ducts. Further back they are limited to lateral areas of pre-cerebral region ( Figures 16 View Figures 16 and 17 , 24 View Figures 20–27 ) and taper to end just even with anterior portion of brain (Figure 17).
Submuscular glands. Though not further described, Bürger notes, “Our species exhibits another characteristic, which separates it from Drepanophorus and brings it closer to Amphiporus . It is in the possession of numerous subepithelial gland cells, which are arranged in the manner of the cephalic glands in the tip of the head, and have nothing to do with the frontal gland.” The glands that Bürger referred to as subepithelial are now known as submuscular because they lie among the longitudinal muscles entirely inside the circular layer. From this statement it may be inferred that submuscular glands are present as in most other benthic members of the Cratenemertidae and are situated peripherally near the body wall as is shown for the cephalic glands in Figure 5 View Figures 4–6 . In neotype specimen submuscular glands situated mostly in anterior portion of pre-cerebral region as relatively small packets among longitudinal muscles close to circular layer (typically deep red with polychrome stains of Mallory type).
Cephalic grooves. “The cephalic grooves are prominent and were distinguished through a dark yellow colour ( Figure 4 View Figures 4–6 ).” In slides of neotype specimen, epithelium of this area is separated from dermis but otherwise intact. Anterior cephalic groove shows characteristic secondary grooves and rugae which have been sectioned transversely where the groove runs down the side of the head (Figure 17). Anterior cephalic grooves in this species have same compound structure found in other benthic members of family. Posterior grooves small and shallow, meeting in posteriorly directed point in dorsal midline.
Body wall, musculature and integument. “The epithelium is preserved only in small shreds and is filled with very slim, almost rod-shaped gland cells, whose contents stain blue with haematoxylin.”
“The dermis is extraordinarily thick ( Figures 7 View Figures 7 and 8 , 10, 11, 13). It consists of numerous concentric, crinkled layers and contains unusually numerous small nuclei. In its outermost layers there are also numerous small capsule-like cell bodies. In an unstained tangential section of the dermis these elements are noticeable as oval structures, which under polarization contain a shining corpuscle. In haematoxylin-stained sections, except for the shining core which represents the nucleus, to all appearances secretion-like substance is strongly tinged. The same either consists of granules rather uniformly distributed in the entire capsule or they fill only the section of the capsule toward the body wall. Then they appear compressed and have the granular structure missing. In this state, which is abundant, the capsules entirely resemble tiny eyes ( Figure 6 View Figures 4–6 ). What we have demonstrated in reality, I am not able to say. There remains nothing to add except their distribution in the body. One readily determines them in greatest abundance in the entire circumference of the body in the nephridial region, only in the dorsum and sparser in the mid-stomach region, or they are missing entirely in the tip of the head and in the caudal sections.” What Bürger’s Figure 2 View Figures 1–3 (C) actually shows is submuscular glands, the secretions of which often appear granular.
Epithelium quite thick as in most members of family ( Figures 14 View Figures 14 and 15 –19). Cells have characteristic U-shaped bases that embed in outer side of dermis. Dermis is about same thickness as circular muscle layer and has well-developed fibrous structure characteristic of benthic members of the family (Figures 17–19).
“The body wall musculature is, as in the rest of the littoral drepanophorans, very powerfully developed. Even the circular muscle layer is strongly developed. Between the latter and the longitudinal muscle layer a diagonal muscle layer has interposed itself, of which we have convinced ourselves via superficial [tangential?] preparations.”
Circular muscle layer powerfully developed. In pre-cerebral region nearly the same thickness as dermis, but in brain and foregut regions and further posteriorly, about one-third to one-half the thickness of dermis.
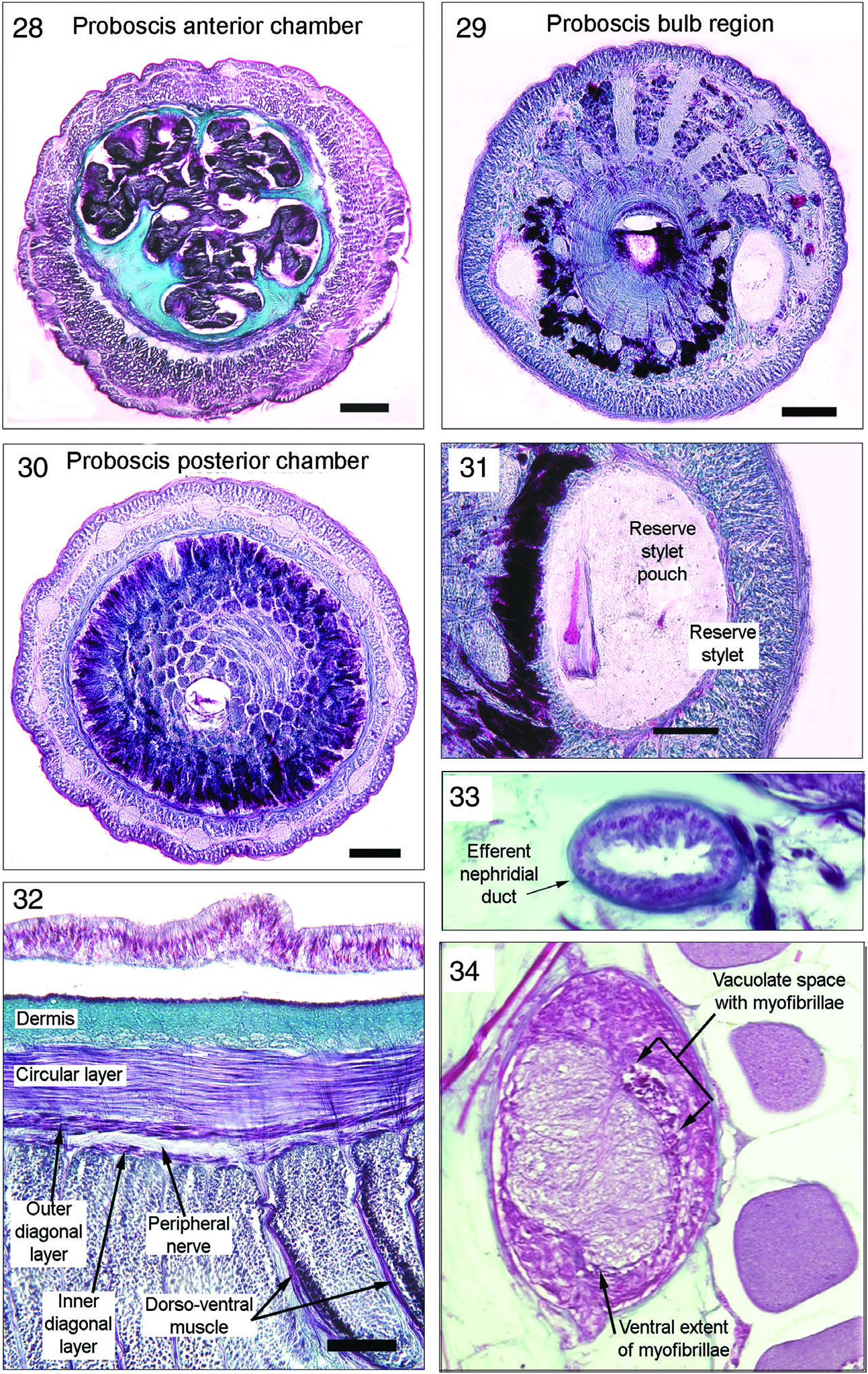
Between circular and longitudinal layers is non-fasciated diagonal muscle layer. In most regions of the body unusually thick and well-developed – perhaps the most heavily developed in the family – of about one-third to one-half the thickness of circular layer. In intestinal region are many areas where it consists of an outer layer in one oblique orientation and an inner layer in the other. Frequently the two layers appear separated by one of the peripheral nerves which arise from the lateral nerve cords and run between the longitudinal muscle wedges to bend into the body wall and provide the innervation of these muscles ( Figure 32 View Figures 28–34 ).
Longitudinal muscle layer consists, as throughout Cratenemertidae , of primary structure of distinct wedges separated from one another by spaces through which pass nerves and dorsoventral muscles ( Figures 6–12 View Figures 4–6 View Figures 7 and 8 View Figures 9 and 10 View Figures 11–13 , 14 View Figures 14 and 15 –19). In anterior parts of body wedges are quite deep and powerful ( Figures 7–11 View Figures 7 and 8 View Figures 9 and 10 View Figures 11–13 ) and less deep, but still powerful, posteriorly ( Figures 12, 13 View Figures 11–13 , 41 View Figures 35–42 ). Each wedge enclosed within very thin fascial membrane and has secondary substructure of honeycomb of fascia-bounded spaces within each of which lie several longitudinal muscle fibres. This wedge structure coupled with extensive dorsoventral musculature would readily accommodate the posterior body attening needed for limited anguilliform swimming. Figures 12, 13 View Figures 11–13 shows that the posterior body of specimen was preserved in much attened condition that would ideally adapt it for such swimming motions as are known from Nipponnemertes punctatulus and Nipponnemertes fernaldi .
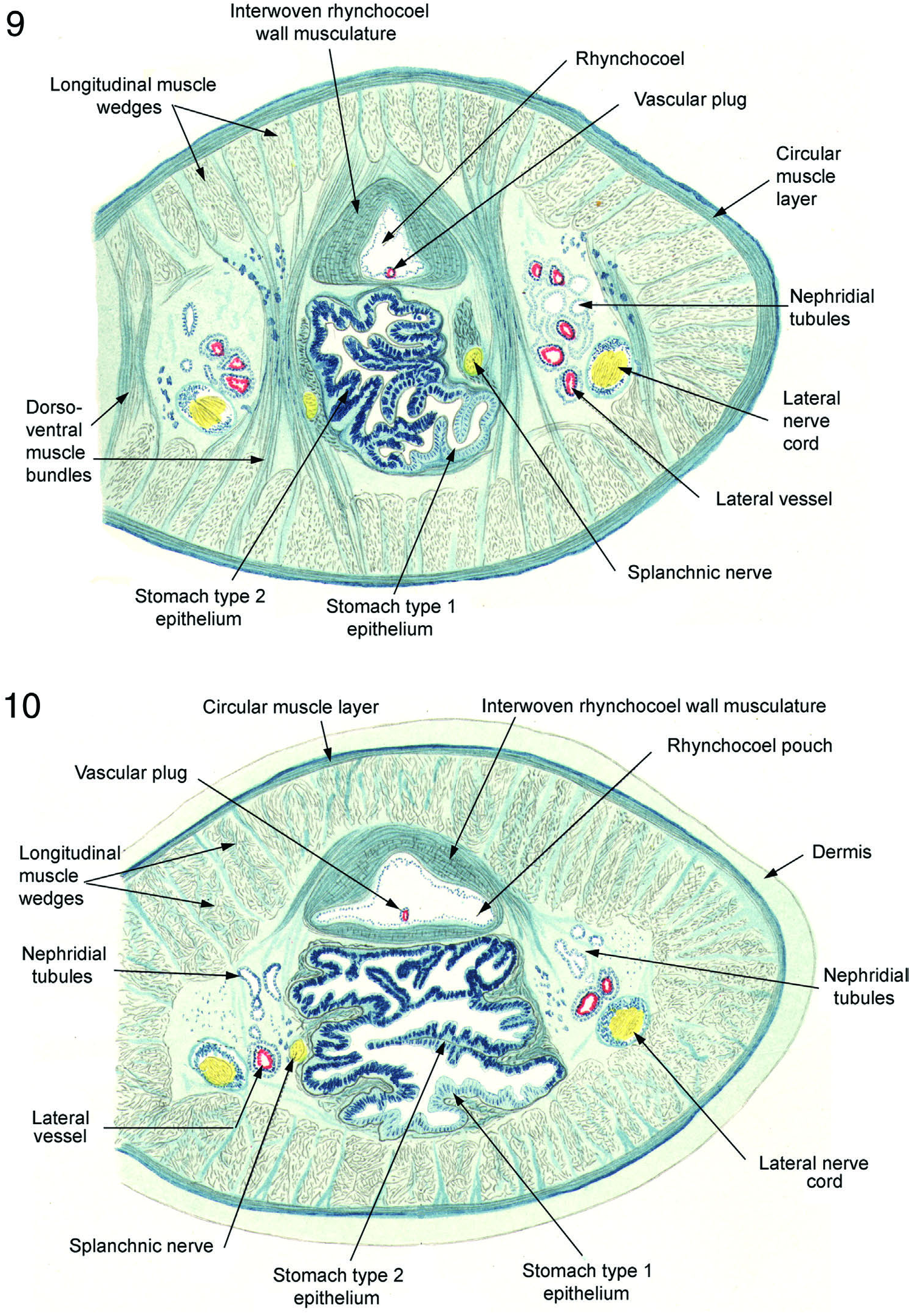
“The parenchyma and the dorsoventral musculature are as is the case in Drepanophorus crassus and Drepanophorus spectabilis . The strong dorsoventral muscle bundles, which separate the rhynchocoel and foregut from the blood vessels and nephridial tubules next to the lateral nerve cords in the nephridial region, are somewhat striking ( Figure 9 View Figures 9 and 10 ).”
Dorsoventral muscles very well developed, arranged in substantial bundles in the foregut region ( Figure 9 View Figures 9 and 10 ) but as small bundles or even stout individual fibres in posterior end of body ( Figures 11, 13 View Figures 11–13 , 32 View Figures 28–34 ). In most parts of body, dorsoventral muscles splay out into fan of fine fibrils as they pass through circular layer and pass as much as two-thirds of the way into dermis ( Figure 32 View Figures 28–34 ).
Pre-cerebral septum is of closed type in which all parts of the body wall longitudinal muscle layer contribute fibres. It wraps around anterior face of brain to enter brain ring as proboscis insertion.
Parenchyma. Bürger’s figures show limited amount of parenchyma anterior to brain ( Figures 5 View Figures 4–6 , 7 View Figures 7 and 8 ), moderate amount in posterior brain and nephridial region (Figures 8–11), whereas posterior part of body is more generously supplied ( Figure 12, 13 View Figures 11–13 ). Neotype specimen with same distribution of sparse parenchyma in the pre-cerebral region ( Figure 14 View Figures 14 and 15 , 15), limited parenchyma in foregut region ( Figures 18 View Figures 18 and 19 , 19) and larger amount in the posterior body except where much of space inside body wall musculature is occupied by ripe gonads ( Figures 40, 42 View Figures 35–42 ).
Rhynchodaeum and rhynchocoel. Bürger’s account states that mouth and rhynchocoel open together. From this it is probably reasonable to infer that the condition is similar to that which he shows for Valdivianemertes stannii (see Amphiporus stanniusi [ sic] Bürger, 1895). Sketch of neotype specimen ( Figure 2 View Figures 1–3 ) shows rhynchostome located subterminally as in with V. stannii and several other members of family. This location and juncture of oesophagus and rhynchodaeum close behind rhynchostome are confirmed by the slide series in the anterior head region ( Figures 14 View Figures 14 and 15 , 23 View Figures 20–27 ).
Bürger’s figure shows powerful rhynchodaeal sphincter lying in pre-cerebral region anterior to septum ( Figure 5 View Figures 4–6 ). However, rhynchodaeum itself is of small diameter, but he gives no details regarding the histology of its lining. In neotype specimen rhynchodaeal sphincter is of considerable length and extraordinarily powerful development – perhaps greater than any other member of the family ( Figure 14 View Figures 14 and 15 ). Rhynchodaeum lined with a tall columnar ciliated epithelium which would atten considerably when the rhynchodaeum expands to contain everted proboscis.
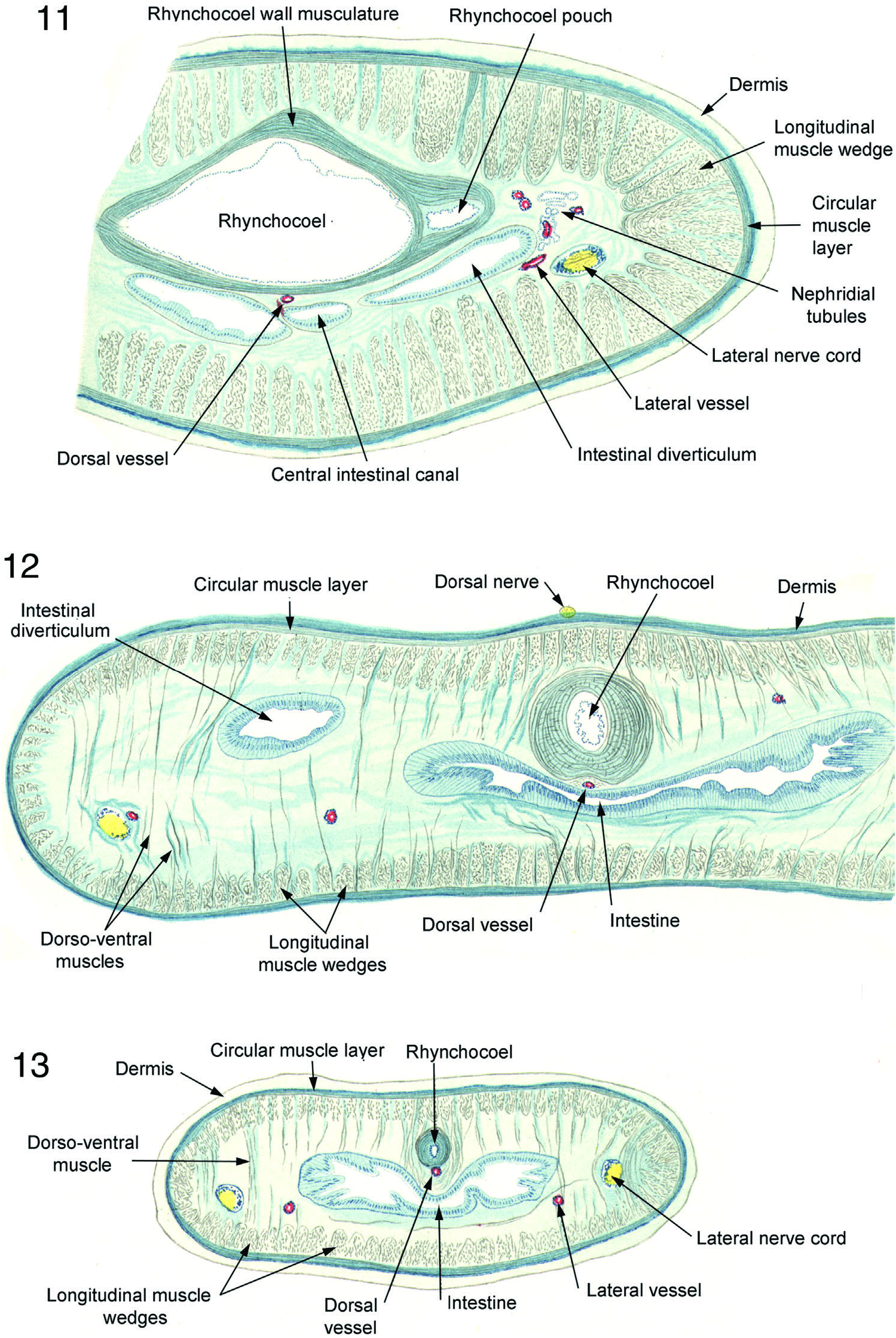
“The rhynchocoel reaches posteriorly to the tip of the body and ends near the anus. The wall of the rhynchocoel comprises an extraordinarily thick muscle layer, which as with other littoral drepanophorans, exhibits the characteristic that the composition of circular and longitudinal fibres are not formed of separate layers but rather are tangled together, indeed in the manner that toward the periphery the circular and medially the longitudinal fibres predominate. Inside, the rhynchocoel is lined with a moderately thick gelatinous layer, which especially in the nephridial region produces two swellings dorsally on the right and left ( Figure 11 View Figures 11–13 ). The gelatin forms the ground layer for the attened epithelium whose cells are remarkably small, so that their nuclei adjoin close to one another.”
In neotype specimen structure of rhynchocoel wall musculature shows with particular clarity in posterior region as a single composite layer comprised of numerous alternating strata of circular and longitudinal muscle fibres that do not appear to be as heavily interwoven as most species of family ( Figure 38 View Figures 35–42 ).
“It has already been emphasized that the rhynchocoel of D. valdiviae is quite significant in that it differs from the hitherto known littoral species of Drepanophorus in having the pouches limited to the anterior section of the body (that is, essentially in the nephridial region). These pockets outpouch at regular intervals opposite one another and represent moderately deep sacs, or better said protrusions, which possess a very thick wall ( Figures 9 View Figures 9 and 10 , 11 View Figures 11–13 ).”
It is clear from the above that Bürger was regarding the rather short, plump thickwalled outpouchings of the rhynchocoel wall in V. valdiviae as homologues of the long, slender thin-walled diverticula of the drepanophorans. However, Stiasny-Wijnhoff (1923) correctly recognized the homology with the pouches of Bürger’s Amphiporus stanniusi and the distinction respectively from Drepanophorus and Amphiporus and established the genus Valdivianemertes for the two species (cf Crandall 1993).
In neotype pouches are a series of paired lateral protrusions of rhynchocoel wall. Walls of protrusions throughout are same thickness as rhynchocoel wall and of identical structure ( Figure 35–37 View Figures 35–42 ). Their length from the outside of the rhynchocoel is about equal to rhynchocoel diameter. They first appear in mid-stomach region and continue posteriorly only to level of mid-caecum a little before pyloric–intestinal junction
Proboscis. Proboscis missing in Bürger specimen. Had it been present Bürger would have been most unlikely to have attributed his specimen to Drepanophorus .
Neotype proboscis consists of typical monostiliferous hoplonemertean anterior chamber, bulb region, and posterior chamber ( Figures 28–30 View Figures 28–34 ). Anterior chamber very long and in everted position is covered by well-developed papillae supported by radial extensions of the connective tissue layer located between the epithelium and musculature. Musculature comprises an outer circular, central longitudinal and weak inner circular layer, often with only a few fibres. Twelve large proboscis nerves lie in longitudinal layer and are connected by a neural sheath that divides longitudinal layer into thick outer and thin inner portion next to the inner circular muscle fibres ( Figures 28, 30 View Figures 28–34 ). Bulb region contains central armature consisting of the central stylet and basis and two reserve stylet pouches containing two and three stylets, respectively ( Figures 29, 30 View Figures 28–34 ). Reserve stylets about 65 Μm in length. Posterior chamber of smaller diameter than anterior and lacks circular layer between epithelium and longitudinal muscles, lined with secretory epithelium with secretions passing through bulb duct to bathe central stylet with venomous secretions as an aid in prey capture. Longitudinal muscle fibres continue beyond end of posterior chamber to form proboscis retractor muscle which connects to the musculature of the rhynchocoel wall.
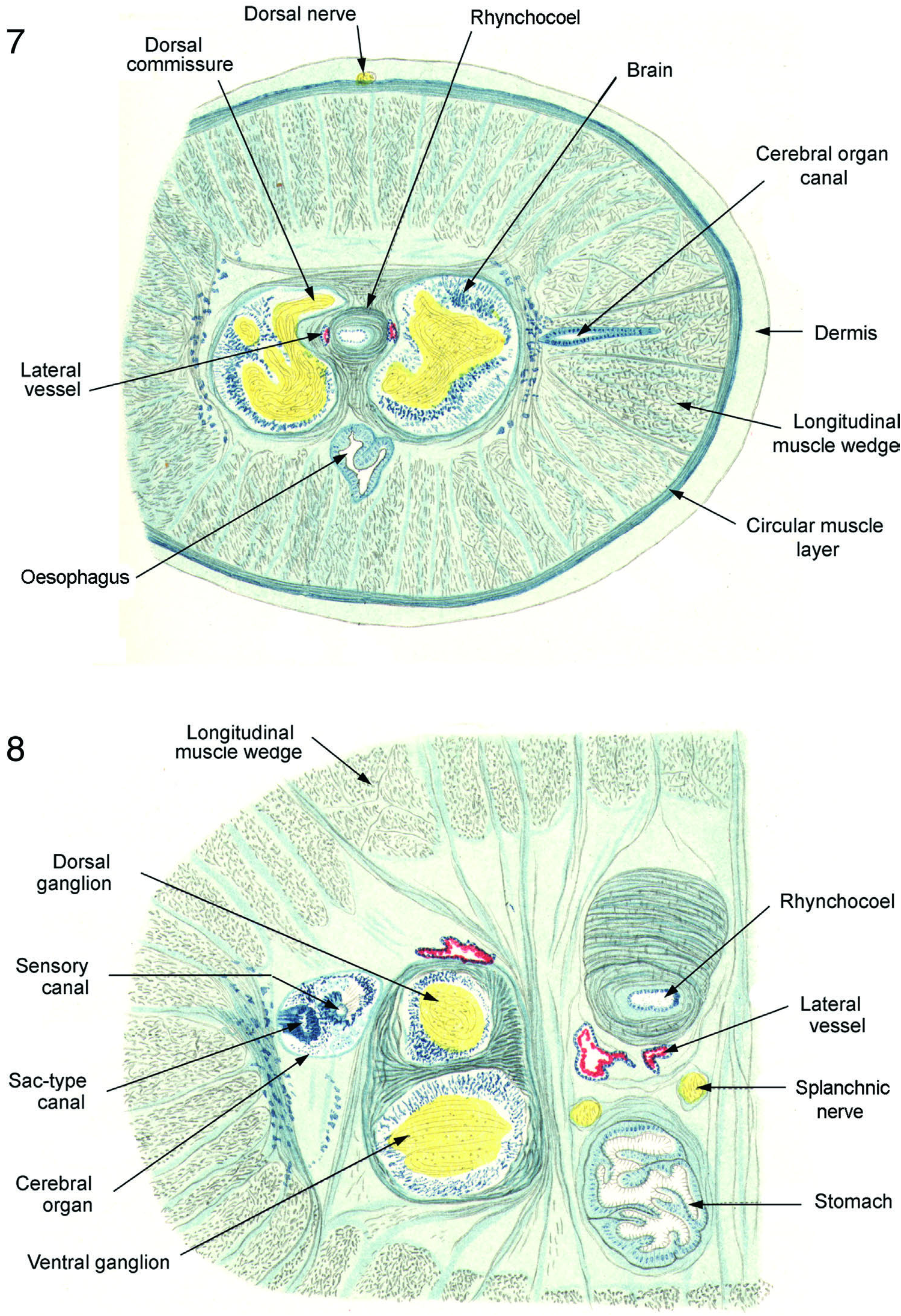
Nervous system. “The brain and lateral nerve cords likewise show, in relation to the ganglion cell layer, proportions as I have described earlier especially with reference to Drepanophorus crassus and Drepanophorus latus . The lateral nerve lies ventrally, but for a Drepanophorus strikingly lateral. Dorsal and splanchnic nerves were observed.”
In neotype brain somewhat below average size for Cratenemertidae , right and left halves of more rounded shape than most other benthic members of the family. In anterior and middle portions, no external indentation between dorsal and ventral ganglia, even in mid-brain area where fibre cores become separated. Dorsal and ventral ganglia become completely separated a little before posterior termination of dorsal ganglia.
Dorsal commissure joins dorsal ganglia near their anterior ends ( Figure 7 View Figures 7 and 8 ). Ventral commissure of somewhat smaller than average size and arises from lower medial portions of ventral ganglia.
Relatively large dorsal nerve runs from anterior brain region ( Figure 7 View Figures 7 and 8 ) through posterior intestinal region ( Figure 12 View Figures 11–13 ) to near end of body. It lies inside epithelium in distal side of dermis. Pair of large splanchnic nerves arise from near the rear of medial sides of ventral ganglia and run along dorsolateral aspect of stomach (Figures 8, 9, 19, 27) and a pair of large dorsolateral nerves arise from dorsomedial portion of dorsal ganglia ( Figure 25 View Figures 20–27 ) and lie above level of brain at proximal edge of longitudinal muscle layer (Figure 19).
Lateral nerve cords of neotype have connective tissue band that lies between fibre core and neuroganglionic cells on the medial side and is characteristic of all Cratenemertidae . Both the connective tissue and the myofibrillae originate outside the brain a little ventral and posterior to ventral commissure and enter medial side of the ventral ganglion ( Figure 26 View Figures 20–27 ). Connective tissue band has usual vacuolate space in dorsal one-quarter to one-third, which is filled with an unusually large number of myofibrillae ( Figure 34 View Figures 28–34 ). There are also some myofibrillae farther ventrad lying tightly against lateral side of connective tissue band next to fibre core of cord. Nerve cords occupy customary positions for all benthic cratenemertids throughout their course along the body. While Bürger’s comment on the position of nerve cords relative to drepanophorans is quite true, his assumption that this taxon was a drepanophoran is not. Neither Bürger’s text nor illustrations provide details of finer structure of nerve cords, such as, connective tissue elements and myofibrillae. Internal structure of the nerve cords of cratenemertids, amphiporids and drepanophorans are very different. Internal structure of neotype nerve cord places it unquestionably in Cratenemertidae and further refutes Bürger’s placement among drepanophorans. Bürger gave no details regarding the posterior anastomosis of the nerve cords.
Posterior section series of neotype ends slightly before end of body and just ahead of posterior anastomosis of nerve cords. However, posteriormost sections show nerve cord migration upward and inward toward an anastomosis which will lie above gut.
Bürger provided no information as to neurochord cells and neurochords being present or absent. Neotype specimen has neither neurochord cells nor neurochords, and such are not known for any members of Cratenemertidae .
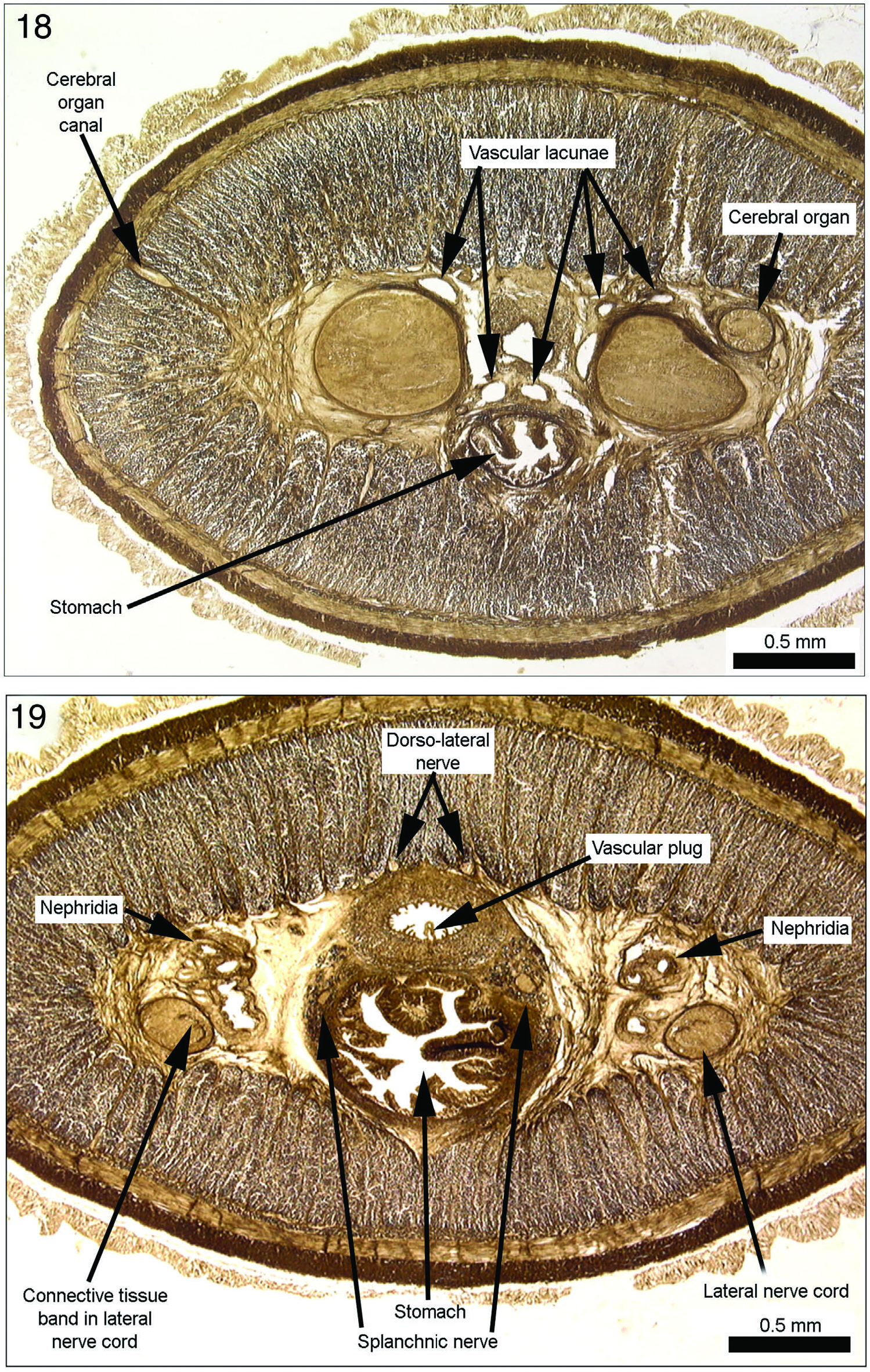
Cerebral organs. “Likewise cerebral organs are found, to which the presence of cephalic grooves points. They lie in the posteriormost brain region lateral to the dorsal ganglia and close against them (Figure 8). They are remarkably small and lack the sac-like enlargement, which for the cerebral organ of Drepanophorus is otherwise characteristic. The cerebral organs of D. valdiviae resemble, therefore, more those of Amphiporus and accord almost completely overall with those of Prostoma .”
Cerebral organs lie beside dorsal ganglia in posterior brain region, though not tightly against ganglia as is the case with many members of the family. Also, if one credits Figure 10 as being accurate, they are of moderate rather than small size. In neotype because of moderate brain size they are comparatively moderate in size. Moreover, a laterally placed sac-like canal separate from the more medially located sensory canal is clearly shown in Bürger’s figure (Figure 8) and is present in the neotype ( Figure 26 View Figures 20–27 ). While cerebral organ, as figured in illustration, would indeed be relatively small for a drepanophoran, it is of moderate or somewhat below average size for benthic cratenemertids. As a presumed drepanophoran Bürger was expecting a sizeable sac, but some Cratenemertidae have only a lateral bulge in anterior canal lined with sac-like epithelium, while sensory epithelium lies in medial half-round portion of canal. Hence, in at least a few species there is not a separate protuberance lined in its entire periphery with sac-like epithelium. Bürger shows cerebral organ canal ( Figure 7 View Figures 7 and 8 ) as passing almost straight laterally from anterior brain toward the body wall. Canal is also unusual in that it passes somewhat above the horizontal midline and angles slightly upward as it passes toward the body wall (Figures 17, 18 and 8A), whereas in most benthic cratenemertids it opens in a ventrolateral position, usually just ahead of the septum. Though not as clearly stated as it might have been, Bürger’s comment recognized association between cerebral organ canal and anterior cephalic groove. Unfortunately he gave no information for glandular structure of organ or for cerebral organ nerves.
Cerebral organ nerve bundle arises from top of fibre core of dorsal ganglion near its end and passes through lateral face of ganglion and then almost directly into dorsomedial portion of cerebral organ ( Figure 26 View Figures 20–27 ). Because of staining in anterior section series, it was not possible to determine the finer details of branching of nerve bundle and to trace various components.
Blood vascular system. “The blood vessels are as is universal in the Metanemerteans. The dorsal vessel is enclosed within the rhynchocoel only in the anterior nephridial region ( Figures 9 View Figures 9 and 10 , 10).”
Vascular system of standard hoplonemertean architecture of pre-cerebral vascular loop and three post-cerebral vessels, two lateral and one mid-dorsal. Pre-cerebral vascular loop anastomosis occurs far anterior (about 250–300 Μm behind the tip of the head) above rhynchostomodaeum ( Figure 21 View Figures 20–27 ). Lateral portions of loop ank rhynchodaeum ( Figures 5 View Figures 4–6 , 23, 24 View Figures 20–27 ). Left side first bifurcates into two branches and sends off third branch at some distance below the other two, right side trifurcates into three branches ( Figures 14–16 View Figures 14 and 15 View Figures 16 and 17 ). Branches of each trifurcation re-anastomose in region of pre-cerebral septum before the two vessels of each side of the loop pass posteriorly between right and left brain halves and the rhynchocoel on the two sides of the brain ring (Figure 17).
Just after entering the brain ring the vessels form the unusual feature of sending off several lacunae that lie around the anterior proboscis sheath and extend along the dorsomedial and ventromedial portions of the brain (Figures 17, 18). Lacunae irregular in shape and size and extend back only to about the level of the middle of cerebral organs. Although Bürger’s text does not mention these lacunae, they are clearly illustrated in his figure of this region (Figure 8).
Mid-dorsal vessel arises from the left lateral vessel as it exits the brain ring. It rises through rhynchocoel wall to form median vascular plug (Figures 19, 27) which continues posteriorly against ventral inner wall of rhynchocoel to mid-pyloric region of foregut ( Figures 35, 36 View Figures 35–42 ) where it descends through the rhynchocoel wall to assume its position between rhynchocoel and gut ( Figure 37 View Figures 35–42 ). As is usual, vessels show a number of parallel branches in nephridial region (Figures 19, 27, 35) which re-anastomose at its posterior end as lateral vessels move to a position medial to lateral nerve cords. This position of vessels prevails from end of nephridial region to posterior end of body. Transverse connectives not present.
Posterior section series of neotype ends a little before end of body and slightly ahead of posterior anastomosis of blood vessels. However, posteriormost sections show lateral vessel migration upward and inward toward vascular anastomosis which will lie above the gut.
Neither “valves” nor “pouches” present and only an occasional individual blood cell seen. Where vessel walls were fairly thick and a polychrome stain used, both circular and longitudinal muscle fibrils seen in vessel walls.
Excretory system. “The nephridial tubules are rather extensive in that they reach from the brain into the anterior stomach region and are extensively branched; they twist among the various blood vessels ( Figures 9 View Figures 9 and 10 , 10). There is probably only a single nephridiopore on each side. They are located in the anterior intestinal region and they are a continuation of the posterior end of the nephridial tubules. The pores lie laterally on each side somewhat near the dorsal surface.”
Neotype nephridia begin a little posterior to cerebral organs and above transition of ventral brain lobes into lateral nerve cords (Figures 19, 27, 35) and extend posteriorly to mid-pyloric region. Posteriorly they extend downward into space between stomach and lateral nerve cords. From Bürger’s illustrations, nephridial tubules appear to be constructed of customary cuboidal epithelium and this is true of neotype. They are interspersed around and among small blood vessels with which they are associated. Efferent ducts in neotype begin at posterior end of mass of nephridial tubules and extend posteriorly beyond pyloric–intestinal junction well into intestinal region ( Figure 33 View Figures 28–34 ). They lie immediately adjacent to and for the most part dorsal to lateral nerve cords. Ducts then migrate laterally and a little ventrally to exit through body wall somewhat below horizontal midline.
Bürger’s statement about dorsal position of the efferent nephridial ducts may therefore be questioned. In no species of benthic Cratenemertidae are efferent nephridial ducts (nephridiopores) known to open above horizontal midline. It is not known whether Bürger had sectioned this portion of specimen and he might have been expressing an assumption about their position. In the vast majority of cratenemertids they open ventrolaterally a little anterior to the pyloric–intestinal junction. The epipelagic forms represent an exception where the efferent ducts occur in the mid-body region and open laterally.
Alimentary canal. “The mouth and rhynchodaeum open together. The oesophagus, which is without gland cells, is broad and long; it reaches beyond the brain posteriorly. The stomach is very strongly developed and occupies almost the entire nephridial region ( Figures 9 View Figures 9 and 10 , 10). The development of a caecum is nearly suppressed. The central tube of the intestine is narrow; however, the diverticula are extraordinarily long ( Figures 11, 12 View Figures 11–13 ).”
Bürger’s statement that mouth and rhynchodaeum open together, coupled with finding in neotype that rhynchostome is distinctly subterminal, makes it reasonable to infer that the condition was very similar to that shown for Valdivianemertes stannii [see Amphiporus stanniusi ( Bürger, 1895) ]. In neotype, rhynchostomodaeum extends only a few sections from rhynchostome before separation of rhynchodaeum and oesophagus ( Figures 14 View Figures 14 and 15 , 15, 23).
Oesophagus lined with low non-glandular epithelium and transitions into stomach posterior to ventral brain commissure. Stomach large with numerous folds and rugae ( Figures 18 View Figures 18 and 19 , 19, 27). Anterior parts of neotype stomach have type 1 stomach epithelium (the gland cells of which have granular secretions), which gradually gives way to type 2 stomach epithelium (the gland cells of which have primarily mucoid secretions) posteriorly.
Although Bürger’s text does not mention the stomach epithelium being regionalized, this condition is clearly illustrated ( Figures 9 View Figures 9 and 10 , 10). Regionalization of stomach epithelium has been noted by Gibson and others for several species of hoplonemerteans. In Bürger’s 1909 figures the type 1 epithelium occupies a relatively small area on oor of stomach while the much more extensive type 2 epithelium occupies middle and upper areas. Such regionalization of stomach epithelium is found in many, if not most, Cratenemertidae and indeed prevails in neotype. In neotype a large bulge of stomach protrudes into the expanded anterior pylorus ( Figure 36 View Figures 35–42 ); however, this is regarded as a fixation artefact and has been seen in other cratenemertid specimens that have not achieved full anaesthetization of innermost structures before fixation.
Bürger’s text indicates that caecum would be short. However, his text provides no account of the extent of diverticula or anterior pouches, and his figures do not include this region of specimen. As noted, central canal of intestine is of small diameter, but diverticula are large and extend far laterally. In neotype, caecum short with small diameter and only three or four pairs of lateral diverticula, all of which short. Two anterior pouches on each side ( Figure 37 View Figures 35–42 ) but all quite short, i.e. less than 100 Μm. Posterior to pyloric–intestinal junction central intestinal tube is of modest diameter, and lateral diverticula are equal to it in height ( Figure 41 View Figures 35–42 ). Diverticula are of moderate length, generally 2 to 21 / 2 times as long as diameter of central tube, and curve dorsolaterally to about horizontal midline of the rhynchocoel.
Bürger gave no descriptive information for hindgut. Posterior slide series of neotype ends a few sections before beginning of the hindgut, but if specimen is similar to virtually benthic cratenemertids, it will have a short rectum with an epithelial lining distinctly differentiated from that of intestine, and rectal tube will be surrounded by circular muscle fibres separate from the body wall arranged into some type of sphincter.
Reproductive system. Gonads were not developed in Bürger’s specimen.
Neotype female with moderate-sized ova in late stage of development with many being fully mature ( Figures 38–40 View Figures 35–42 ). Ovaries in anterior intestinal region positioned laterally but in more posterior areas as many as seven ovaries may be seen in a single section and nearly fill space between body wall and gut ( Figure 42 View Figures 35–42 ). Number of oocytes varies but generally 40–45 oocytes in slightly different stages of maturity may be counted in section of a single ovary ( Figures 40, 42 View Figures 35–42 ). Mature oocytes are from 120 to 140 Μm in diameter and are surrounded by jelly coat 10–20 Μm thick. Oviducts open through dorsolateral body wall ( Figure 39 View Figures 35–42 ), but are not yet present in most ovaries, suggesting that most ova are just short of final maturity.
There is no indication that sexes are other than separate. There are numerous fine meshwork muscles contained within rather thin walls of ovaries. “Nuage bodies” are not present, although ova are at the stage of development where such material would appear if present.
Habitat
Bürger’s specimen was collected by trawl from a bottom with a depth of 155 m off South Africa. Neotype was collected by Agassiz trawl from a 602 m bottom of muddy brown gravel (gravel 9.3%, sand 27.4%, silt 63.4%); M.O. 4.64%; redox ( E h) 240.5 in the Bellingshausen Sea, Antarctica.
Distribution
Bürger’s original single specimen was taken at RV Valdivia Station 104, 35 ◦ 16.0 S, 22 ◦ 26.7 E, approximately 200 km east of south east by east of Cape Agulhas , South Africa. The neotype specimen was taken by the RV Hespérides (BENTART 2003 cruise) at Station 13, in the Bellingshausen Sea, 69 ◦ 49 34 S, 77 ◦ 49 92 W, Antarctica ( Figure 1 View Figures 1–3 ) GoogleMaps .
| RV |
Collection of Leptospira Strains |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Valdivianemertes valdiviae ( Bürger, 1909 )
| Uz, Silvia de la, Anadón, Nuria & Crandall, Frank B. 2010 |
Valdivianemertes valdiviae
| Stiasny-Wijnhoff 1923 |
Drepanophorus valdiviae Bürger 1909
| , Burger 1909 |