Liolaemus gardeli, Verrastro & Maneyro & Da Silva & Farias, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4294.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:3B090754-F7F4-4F1F-8037-253DA8B3D463 |
|
DOI |
https://doi.org/10.5281/zenodo.6033753 |
|
persistent identifier |
https://treatment.plazi.org/id/FC6D8796-FFE0-5A34-FF1F-6398BB20DB2C |
|
treatment provided by |
Plazi |
|
scientific name |
Liolaemus gardeli |
| status |
sp. nov. |
Liolaemus gardeli sp. nov.
Holotype. ZVC-R 6823 , adult male collected by L. Verrastro, R. Maneyro, and G. Scaron on February 21, 2014, in an area of sand dunes in the Tacuarembó department (31°58'43''S, 55°31'18.7''W), Uruguay. GoogleMaps
Paratypes. All specimens were collected in the same area and locality: ZVC-R 6824–6831 , collected on February 21, 2014, by L. Verrastro, R. Maneyro, and G. Scaron; UFRGS 6630–6637 collected on March 27, 2013, by L. Verrastro and G. Scaron.
Diagnosis. Liolaemus gardeli is a member of the wiegmannii group because it presents lorilabial scales smaller than the supralabials and narrow (longer than wider) supralabial scales. The mental scales are in contact with the sublabials. The infralabials are concave. Liolaemus gardeli differs from other Liolaemus spp. of the wiegmannii group by its large, blood-colored stain at the gular region in males that reaches the rostral, infralabial, and supralabial scales. There are red dots on the scales of the posterior region of the ear, the canthal scales, the superciliary, and the inner ciliary scales, features totally absent in other species of the L. wiegmanni group. The rostral scale is partially or slightly subdivided by the central postrostral. The scales of the temporal region are smooth, with a slight rugosity on the upper region; there are two large parietals that reach half of the interparietal. The species has a large mental scale. The pattern on the dorsal part of the body presents two mid-dorsal rows of quadrangular-shaped stains, with the darker area being larger than the lighter area, a feature absent in all other species of the L. wiegmanni group. In addition, L. gardeli is one of the smallest species in the L. wiegmannii group.
Liolaemus gardeli has nasal scales (in the shape of a drop) in a dorsal position, with the nostrils occupying half of the scale, and narrower at the anterior end; this differs from L. wiegmannii , which has laterally-directed nostrils, a nasal scale in dorsal position, and the nostril occupying half of the scale. It also differs from L. occipitalis , in which the nostril occupies most of the scale (Etheridge 2000); from L. cuyumhue , in which the nostril is dorsolaterally positioned and occupies less than half of the scale (Ávila et al. 2009); and, from L. azarai , in which the nostril is dorsolaterally positioned (Ávila 2003). Liolaemus gardeli has two rows of lorilabial scales between the subocular and supralabial regions; it differs from L. arambarensis , which has one row of lorilabial scales (Verrastro et al. 2003), L. cuyumhue , which has two to three rows (Ávila et al. 2009), and L. lutzae , which has only one row (Etheridge 2000). The head scales of L. gardeli are smooth, while the temporal scales are smooth and slightly rugose on the upper temporal region; in contrast, the temporal scales are keeled and/or rugose in L. wiegmannii ( Fig. 8 View FIGURE 8 ). The mental scale of L. gardeli is higher than in all other species of the L. wiegmannii group, and the posterior side, which is in contact with the postmental scales, ends at a single point. A frontal scale is present, similar to L. wiegmannii , but differing from L. occipitalis and L. lutzae , which do not have a frontal scale. The rostral scale of L. gardeli is large and slightly or partially subdivided by the central postrostral scale, which differs from all other species of the group; it also differs from L. occipitalis , which has two rows of postrostral scales. The dorsal scales of L. gardeli are imbricate and strongly keeled, resembling those of L. riojanus , L. scapularis , L. lutzae , L. azarai , L. occipitalis , L. arambarensis , and L. wiegmannii , but differing from other species of the group: L. multimaculatus , L. rabinoi , L. cuyumhue , and L. salinicola , which have smooth or slightly keeled dorsal scales. The lateral scales of L. gardeli resemble those of L. occipitalis , L. azarai , L. lutzae , and L. wiegmannii , presenting a common pattern that differentiates the dorsal from the ventral region (Etheridge 2000).
Description of male holotype: ZVC-R 6823 (RML 2778 ( Fig. 9 View FIGURE 9 )
Adult male has 49.98 mm SVL, 11.60 mm HL, 10.20 mm HW, with its entire tail autotomized. There are smooth dorsal head scales, including on the temporal region (upper scales slightly rugose). The rostal is slightly subdivided on its upper part by a central postrostral scale and one row of small postrostral scales continues with the lorilabial. There is a gout-shaped nasal scale in the dorsal position, narrower on the anterior extremity, with the nostril occupying half of the scale. There are six irregular internasals, two rows of small scales between the supralabials and the subocular, a pentagonal interparietal with a pineal eye, and two large parietal scales that reach half of the interparietal. There are four large supraorbital scales and a circumorbital circle with small scales that reach the anterior supraorbitals. Scales of the frontal region are irregular. There is one frontal scale that is larger than the adjacent scales, seven supraoculars (the median longer than wider and larger with no evident supraocular semicircle, and laterally separated from the supraciliaries by small supraoculars), and two canthal scales (the anterior smaller and posterior larger and longer). The orbit is surrounded above by seven elongated, oblique, and overlapped superciliaries. There is (in order) only one simple preocular (as high as long) followed by a short, upper postocular (all with a sharp keel along the superior edge). There are small eyelids, occurring immediately below a zone of small reddish ciliary scales, granular inner ciliaries, outer ciliaries slightly projected, three loreals, 10–12 lorilabials in two complete rows (with the scales of the inferior row larger, longer, and more regular than those from the superior row), and lorilabials separated anteriorly (i.e., the supralabials separated from the loreals) and posteriorly (the supralabials separated from the suboculars). There are five supralabials, all longer than taller, a shorter posterior supralabial followed by a set of small and irregular postlabials. The temporals are smooth, juxtaposed anteriorly, with the four, slightly keeled posteriors. The opening of the auditory canal is oblong, higher than larger, anteriorly bordered by a series of small auricular, slightly projected, convex scales. The mental scale is wider than the rostral (1.85 mm in width by 1.33 mm in length), limited posteriorly by two large and wide postmentals and limited laterally by the first infralabial and the first sublabial. There are five infralabials that contact inferiorly with three to four sublabials, three to four pairs of mental shields that are separated anteriorly by three small scales that diverge posteriorly, and a gular region with smooth, flattened, and imbricate scales. The anterior dorsal nuchals are small and smooth, with 57 dorsal body scales that are strongly keeled and imbricate. There are 56 ventral scales (longer than wide and larger than the dorsal), that are imbricate and smooth); 51 of these scales are around the mid-body. There are keeled, superior lateral scales and smooth inferior lateral scales. The dorsal scales of the limbs are keeled similarly to those of the body scales. The granular posterior ventral scales (the remaining ventrals) are larger than dorsal scales, smooth, and imbricate. There are 16 infradigital scales in the fourth anterior finger and 23 scales in the posterior finger. There are also six pre-cloacal pores.
Color in life: The head is predominantly beige, slightly marbled with scales showing dark spots (brown). Two light brown paravertebral stripes run from the neck to the base of the tail with an internal pattern formed of orange quadrangular spots (three scales wide) with a dark brown posterior spot (formed by one to three scales) ending with a white posterior area (one scale high). The line of brown scales that border the white line is darker than the other scales; such stains are regular along the entire dorsum. The vertebral area (occipital band) is beige and has no vertebral line; however, there is a whitish line of one to two scales in width on both sides of the paravertebral bands. Dorsolateral stripes are similar to paravertebral stripes, but the internal pattern of quadrangular spots is diffuse or incomplete. In the anterior lateral region, these stripes exhibit bluish spots, bordered by a row of orange scales, which are diluted in the posterior region. The area between the paravertebral and dorsolateral stripes is intense orange on the anterior region, decreasing in intensity towards the posterior area. The pre-cloacal pores are orange. Ventrally, the body is white speckled with orange spots on the mid-lateral region extending from the chin to half of the body length. In the posterior gular half, there is a spot of bright red. The mental scale is also bright red, as well as the supralabials, infralabials, sublabials, and rostral, which gives an appearance of it having a painted mouth. The cantal, the superciliaries, and the inner ciliary scales are also a bright red-orange color. This reddishorange color also continues through the granular scales of the base of the anterior limb and is in the shape of a round spot ( Fig. 8 View FIGURE 8 ).
Color in alcohol: The specimens are less intense in color, the cyan is less intense, and the orange color of the pores, sides and dorsum fade to beige over time. The brown color also loses its intensity as do the red colorations. The red-orange spots of the venter fade over time, but the white remains unaltered.
Variation. Morphometric data, pholidosis, and number of pre-cloacal pores are presented in Tables 6 View TABLE 6 and 7 View TABLE 7 . The average size of adult male is 37.96 mm and adult female is 36.26 mm ( Fig. 10 View FIGURE 10 ). Rostral scales may occur on one single large-sized scale, to one single large-sized scale partially divided by the central postrostral. The row of postrostrals vary from aligning in one simple row, to a row in which the central postrostral partially subdivides the rostal. The subocular scale is large and elongated and is fused with the postocular in some specimens. Most specimens have three rows of supraoculars, except for one specimen that had four. Parietal scales vary from two entire, large, and regular scales, to a left scale fused with the supraorbital, semicircular scale. The frontal region is formed by irregular scales in a set of two to four scales, the distal always larger than the others. Specimens have 50–69 scales around the mid-body: 54–63 dorsal scales, 49–64 ventral scales, 13–17 infradigital scales on the fourth finger of the forelimb, and 17–24 scales on the hind limb. Pre-cloacal pores are orange, with six to seven on males and four to five on females ( Tables 6 View TABLE 6 and 7 View TABLE 7 ). There is a color sexual dimorphism. Adult males have the color described previously for the holotype. The areas with red coloration are smaller on smaller males (juveniles), the throat does not have red coloration and the cyan (bluish) spots are small. In contrast, females generally have a fainter coloration, which varies from beige to dun, the dorsal spots are less defined, the orange color is more tenuous, and the spots of the mid-dorsal row are smaller, generally showing one scale width in brown and one scale width in white. The lateral stripes of the dorsal region present a row of diffuse spots and a brown color that is deeper than those in males. The ventral region is immaculate white.
Etymology. This new species is named after the famous Uruguayan tango singer, Carlos Gardel, who died in a plane crash in 1935. Gardel’s birthplace was widely disputed and claimed by Uruguay, France, and Argentina, but recent research has confirmed that Gardel is the illegitimate son of a Uruguayan farmer. According to historical data from the book, “Carlos Gardel –el silencio de Tacuarembó,” authored by Selva Ortiz (1994), Gardel was born in the Tacuarembó department ( Uruguay), in the same region of the type locality of this newly described species.
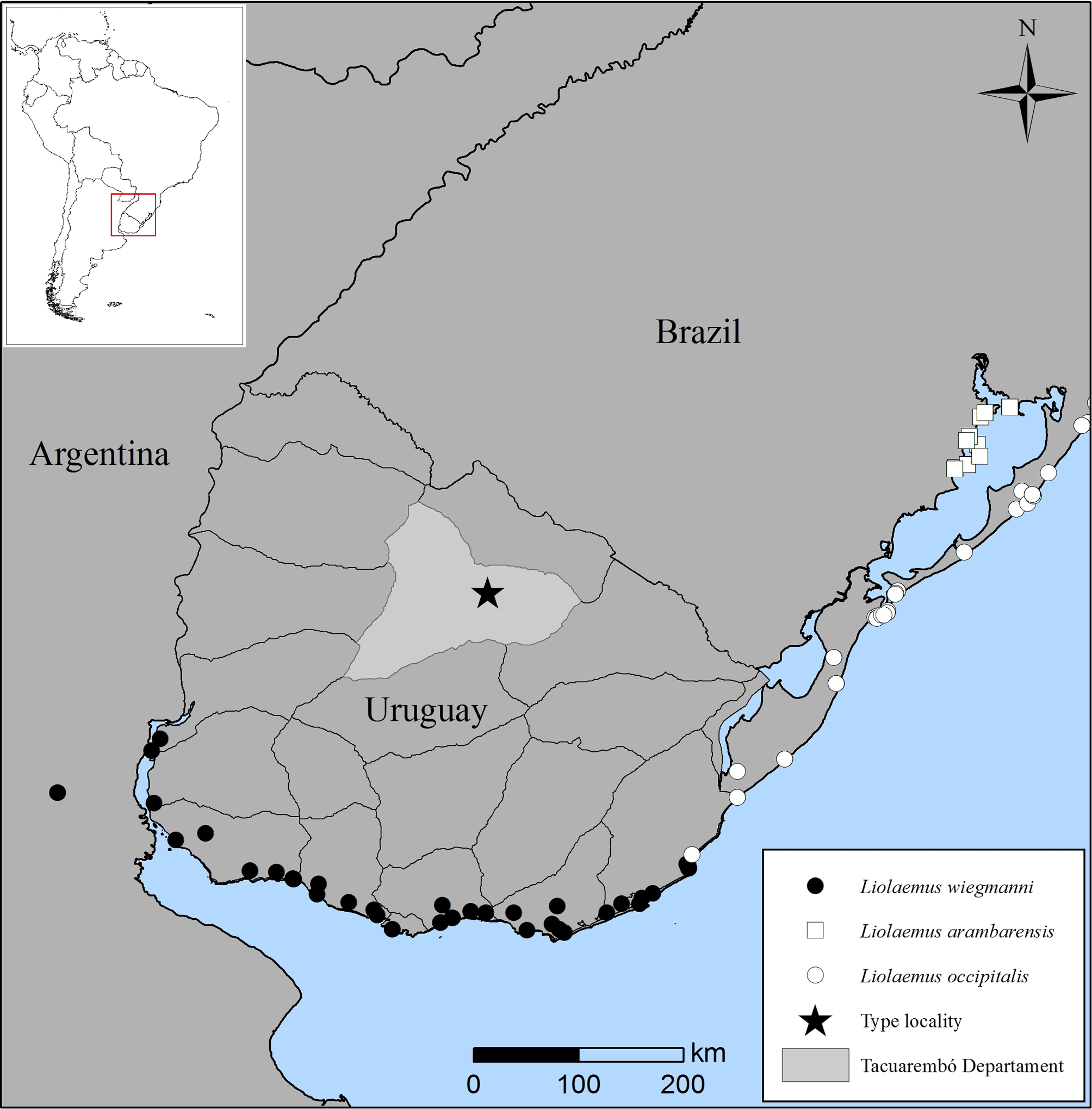
Distribution. The known distribution of L. gardeli is restricted to the type locality, Manantiales Farm (12 km S–SW from Pueblo Ansina), Tacuarembó, Uruguay ( Fig. 1 View FIGURE 1 ).
According to Evia and Gudynas (2000), the region can be characterized as pluvial plains of the upper Negro and Tacuarembó rivers and is associated with the Yaguarí and Caraguatá streams. These waterways are characterized by extensive storm hills, interspersed with marshes, flooded fields, and sand dunes ( Fig. 11 View FIGURE 11 ). Bossi et al. (1975) geologically divided the Tacuarembó River basin into two sections: a lower section deposited in a subaquatic environment, and an upper section of eolic origin, deposited during an arid climatic regime, both of which, according to the authors, would have been deposited during the Triassic Period. According to Sprechmann et al.
(1981), these eolian deposits are characterized by the presence of non-fossilific sandstones generated by the accumulation of eolic dunes during a period of arid conditions.
According to the classification of Köppen, the climate in Uruguay is subtropical humid with hot summers (Cfa). In this region, the vegetation is psammophilous, associated with sand and dunes; vegetation is sparse and xeric. Thus, plant communities are dominated by grasses (such as Panicum racemosum ) Senecio crassiflorus , Hydrocotyle bonariensis , Medicago minima , Dichondra microcalyx , and Calystegia soldanella. Insects and arachnids, such as Allocosa spiders, Coleopterans, and Orthopterans (Evia & Gudynas 2000), occur within the vegetation.
Natural history. Liolaemus gardeli buries easily in the sand to escape predation, as do all other species of the L. wiegmannii group. We have observed some inactive individuals hidden under semi-dry cattle stools at the end of the day. From our in situ observations, this lizard species can easily escape by hiding in herbaceous vegetation, which is thick at the base of the bushes, but it also hides in large dens in dunes. These sand-dune dens seem to belong to armadillos ( Dasypus sp.). Several individuals were observed escaping into these wide and open dens and then stood on hind legs, observing our movement. As soon as an observer approached, they often quickly fled deeply into the dens, but sometimes individuals remained at the burrow entrance. No burrow was observed that could have been constructed by this species, as occurs in L. occipitalis and L. lutzae , for example.
Of the eight open stomachs we examined, seven contained food. From this examination, we concluded that L. gardeli is omnivorous, eating Formicidae , Araneae, Hemiptera, Diptera, Coleoptera , and plants (in three stomachs), including fruits of Cyperaceae , seeds of Poaceae (Cenchrus) . Additionally, an unidentifiable fibrous material was found in one stomach.
Discussion
Our comparison of morphometrics ( Tables 4 View TABLE 4 and 5 View TABLE 5 , Figs. 5 View FIGURE 5 and 6 View FIGURE 6 ) among the species of the wiegmannii group indicates that L. gardeli is one of the smallest species in the group. (However, L. azarai , L. scapularis , and L. arambarensis are also small.) L. gardeli shows a proportionally wider head than the other species. This trait, along with the intense red color of its snout and craw, might help advertise its aggressive behavior when defending territory. (However, this speculation should be subjected to further behavioral research.) Moreover, according to Etheridge (2000), the pattern of the ventral color of Liolaemus is variable and generally not related to the dorsal pattern. Liolaemus gardeli differs from L. wiegmannii and from all other species of the group by the well-marked red ventral color on its gular and the lateral-frontal region of its head ( Fig. 7 View FIGURE 7 ).
Although the genetic distances between L. gardeli and its closest relative, L. wiegmannii , are smaller than between the other species of the group, it should be remembered that Ávila et al. (2009) ( Table 3, p. 49) treated this species as a complex. Thus, the distances between the different populations we analyzed in this study reinforces the idea that there is likely more than one species within the L. wiegmannii complex. Unfortunately, syntypes of L. wiegmannii were collected by other researchers ( Duméril and Bibron 1837) and the type locality “ Chili ” was incorrectly assigned (because this species does not occur in Chile) (Verrastro et al. 2003). Therefore, additional research is needed to restrict the type locality of L. wiegmannii .
TABLE 6. Morphometric variation of the Liolaemus gardeli paratypes (X = mean; Max = maximum; Min = minimum; SD = standard deviation).
| Specimens | Sex | SVL | HL | HW | FLL | HLL | A-G | pores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZVC-R 6823 | male | 48.9 | 11.5 | 9.8 | 17.73 | 26.6 | 21.9 | 6 | ||||||||
| ZVC-R 6824 | female | 29.5 | 7.4 | 6.0 | 10.85 | 16.1 | 13.8 | 5 | ||||||||
| ZVC-R 6825 | female | 44.5 | 10.1 | 8.6 | 16.89 | 23.8 | 20.4 | 5 | ||||||||
| ZVC-R 6826 | female | 48.8 | 10.1 | 8.9 | 17.41 | 24.5 | 23.5 | 4 | ||||||||
| ZVC-R 6827 | female | 46.5 | 10.5 | 9.1 | 16.13 | 24.0 | 21.8 | 5 | ||||||||
| ZVC-R 6828 | male | 41.1 | 9.4 | 7.9 | 14.38 | 23.3 | 19.0 | 6 | ||||||||
| ZVC-R 6829 | female | 30.4 | 8.2 | 6.6 | 11.95 | 17.0 | 12.7 | 4 | ||||||||
| ZVC-R 6830 | female | 29.1 | 7.8 | 6.5 | 10.83 | 16.5 | 12.7 | 4 | ||||||||
| ZVC-R 6831 | female | 27.5 | 7.2 | 5.8 | 11.23 | 16.3 | 10.7 | 4 | ||||||||
| UFRGS 6630 | male | 42.6 | 10.6 | 8.4 | 15.99 | 25.1 | 18.2 | 6 | ||||||||
| UFRGS 6631 | female | 35.7 | 8.4 | 7.4 | 12.85 | 22.0 | 15.4 | 4 | ||||||||
| UFRGS 6632 | male | 22.6 | 6.7 | 5.2 | 9.58 | 14.4 | 9.1 | 7 | ||||||||
| UFRGS 6633 | female | 23.7 | 6.8 | 5.1 | 9.98 | 14.2 | 9.8 | 4 | ||||||||
| UFRGS 6634 | female | 43.4 | 10.0 | 8.2 | 15.28 | 24.5 | 20.4 | 4 | ||||||||
| UFRGS 6635 | female | 36.5 | 9.2 | 7.6 | 13.87 | 22.3 | 16.1 | 4 | ||||||||
| UFRGS 6636 | female | 39.5 | 9.3 | 8.0 | 15.13 | 23.1 | 17.0 | 4 | ||||||||
| UFRGS 6637 | X | male | 45.5 | 37.4 | 11.8 | 9.1 | 9.1 | 7.5 | 16.77 | 13.93 | 27.3 | 21.2 | 19.2 | 16.6 | 7 | 5 |
| X | 37.4 | 9.1 | 7.5 | 13.93 | 21.2 | 16.6 | 5 | |||||||||
| Max | 48.9 | 11.5 | 9.8 | 17.73 | 27.3 | 23.5 | 7 | |||||||||
| Min | 22.6 | 6.7 | 5.1 | 9.58 | 14.2 | 9.1 | 4 | |||||||||
| SD | 8.8 | 1.5 | 1.4 | 2.76 | 4.4 | 4.5 | 1 |
Snout-vent length ( SVL); head length (HL); head width ( HW); forelimb length ( FLL); hindlimb length length ( HLL), axilla-groin distance ( A-G) and number of pre-cloacal pores (pores).
TABLE 7. Variation in scale counts in Liolaemus gardeli paratypes (X = mean; Max = maximum; Min = minimum; SD = standard deviation).
| Specimens | Sex | dorsal scales | ventral scales | mid-body scales | infradigital lamellae anterior | infradigital lamellae posterior | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZVC-R 6823 | male | 57 | 56 | 51 | 16 | 22 | ||||||
| ZVC-R 6824 | female | 58 | 60 | 54 | 16 | 20 | ||||||
| ZVC-R 6825 | female | 57 | 58 | 50 | 16 | 22 | ||||||
| ZVC-R 6826 | female | 61 | 64 | 50 | 17 | 22 | ||||||
| ZVC-R 6827 | female | 60 | 57 | 50 | 15 | 21 | ||||||
| ZVC-R 6828 | male | 60 | 57 | 52 | 15 | 20 | ||||||
| ZVC-R 6829 | female | 61 | 63 | 52 | 16 | 23 | ||||||
| ZVC-R 6830 | female | 59 | 63 | 51 | 16 | 22 | ||||||
| ZVC-R 6831 | female | 57 | 60 | 51 | 14 | 21 | ||||||
| UFRGS 6630 | male | 55 | 52 | 55 | 16 | 23 | ||||||
| UFRGS 6631 | female | 55 | 49 | 53 | 13 | 17 | ||||||
| UFRGS 6632 | male | 58 | 56 | 60 | 16 | 23 | ||||||
| UFRGS 6633 | female | 61 | 61 | 69 | 15 | 24 | ||||||
| UFRGS 6634 | female | 63 | 56 | 59 | 17 | 22 | ||||||
| UFRGS 6635 | female | 62 | 52 | 63 | 15 | 24 | ||||||
| UFRGS 6636 | female | 62 | 53 | 62 | 15 | 22 | ||||||
| UFRGS 6637 | X | male | 54 | 5 9 | 51 | 5 7 | 50 | 5 5 | 15 | 1 5 | 22 | 2 2 |
| X | 5 9 | 5 7 | 5 5 | 1 5 | 2 2 | |||||||
| Max | 63 | 64 | 69 | 17 | 24 | |||||||
| Min | 54 | 49 | 50 | 13 | 17 | |||||||
| SD | 3 | 5 | 6 | 1 | 2 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.