Enenterum kyphosi Yamaguti, 1970
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5154.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:F304EF51-5921-42BC-9B30-C5EAE856471C |
|
DOI |
https://doi.org/10.5281/zenodo.6670922 |
|
persistent identifier |
https://treatment.plazi.org/id/FA5A1A71-3375-CE33-FF50-FA7AFEFEFB09 |
|
treatment provided by |
Plazi |
|
scientific name |
Enenterum kyphosi Yamaguti, 1970 |
| status |
|
Enenterum kyphosi Yamaguti, 1970 View in CoL
( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 )
Type host and locality: Kyphosus cinerascens (Forsskål) , blue sea chub (Centrarchiformes: Kyphosidae ), Hawaii.
Other records: From Kyphosus vaigiensis (Quoy & Gaimard) , brassy chub (Centrarchiformes: Kyphosidae ), collected in Sodwana Bay, KwaZulu-Natal, South Africa ( Bray 1986); from K. vaigiensis , collected from the Ogasawara Islands, Japan ( Machida 1993); from Kyphosus sp. collected from Palau ( Machida 1993).
New localities: Off Amity Point , North Stradbroke Island , Moreton Bay, Queensland, Australia (27°23'53''S, 153°26'15''E); GoogleMaps off Lizard Island, Great Barrier Reef, Queensland, Australia (14°41'10''S, 145°28'15''E) GoogleMaps .
Material studied: 10 whole-mount specimens and two hologenophores ( QM G240075–G240086), from Kyphosus cinerascens collected off Amity Point; five whole mount specimens and three hologenophores ( QM G240087–G240094), from Kyphosus cinerascens collected off Lizard Island.
Representative DNA sequences: ITS2 rDNA, ex K. cinerascens from Amity Point, three identical replicates (two from hologenophores, one from a whole worm), one replicate submitted to GenBank ( ON228451 View Materials ); ex K. cinerascens from Lizard Island, three identical replicates (all from hologenophores), one submitted to GenBank ( ON228452 View Materials ). 28S rDNA, ex K. cinerascens from Amity Point, three identical replicates (two from hologenophores, one from a whole worm), one submitted to GenBank ( ON228454 View Materials ); ex K. cinerascens from Lizard Island, one replicate (from a hologenophore) submitted to GenBank ( ON228455 View Materials ). COI mtDNA, ex K. cinerascens from Amity Point, three identical replicates (two from hologenophores, one from a whole worm), one submitted to GenBank ( ON228462 View Materials ); ex K. cinerascens from Lizard Island, two replicates (both from hologenophores) submitted to GenBank ( ON228460 View Materials – ON228461 View Materials ).
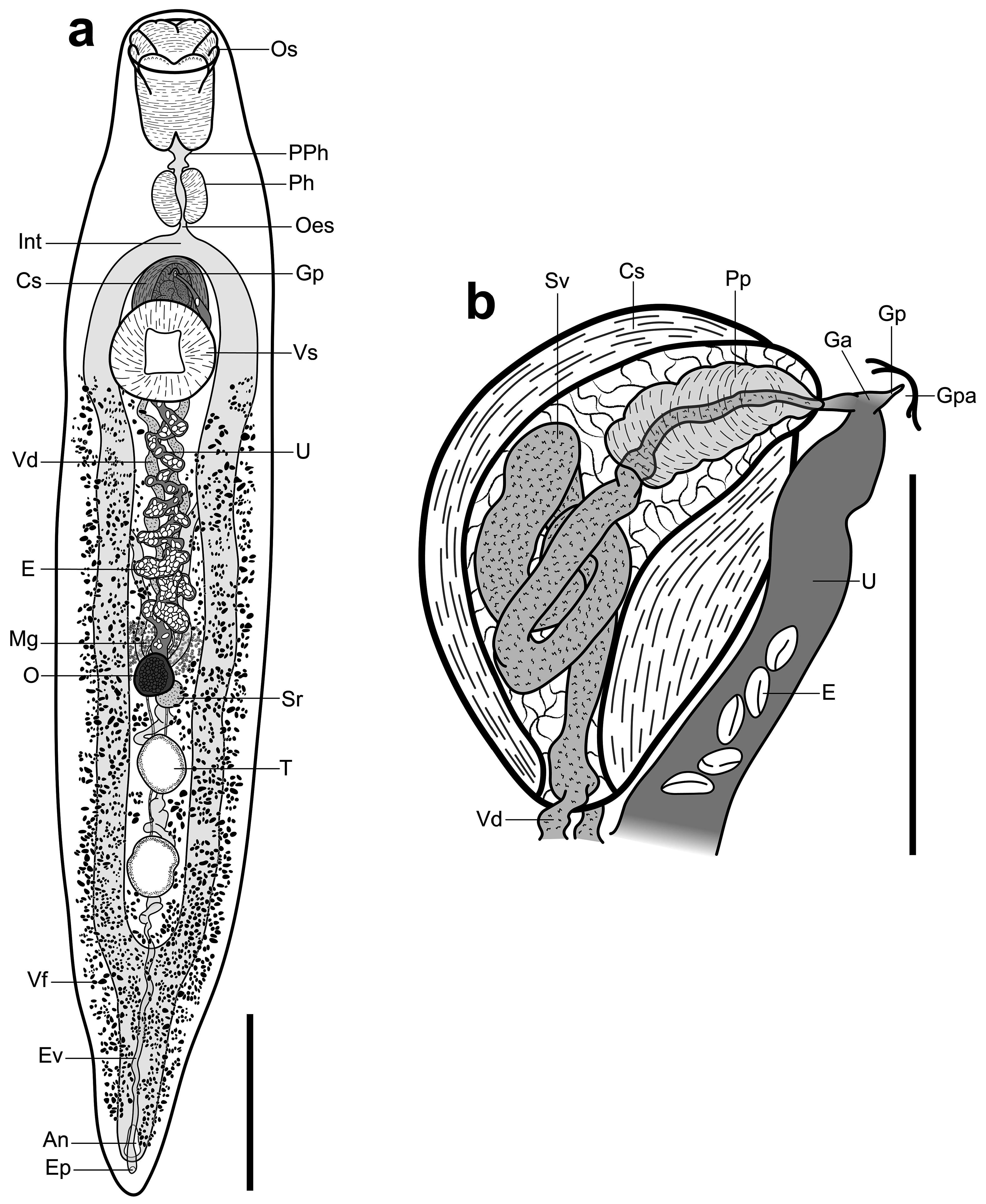
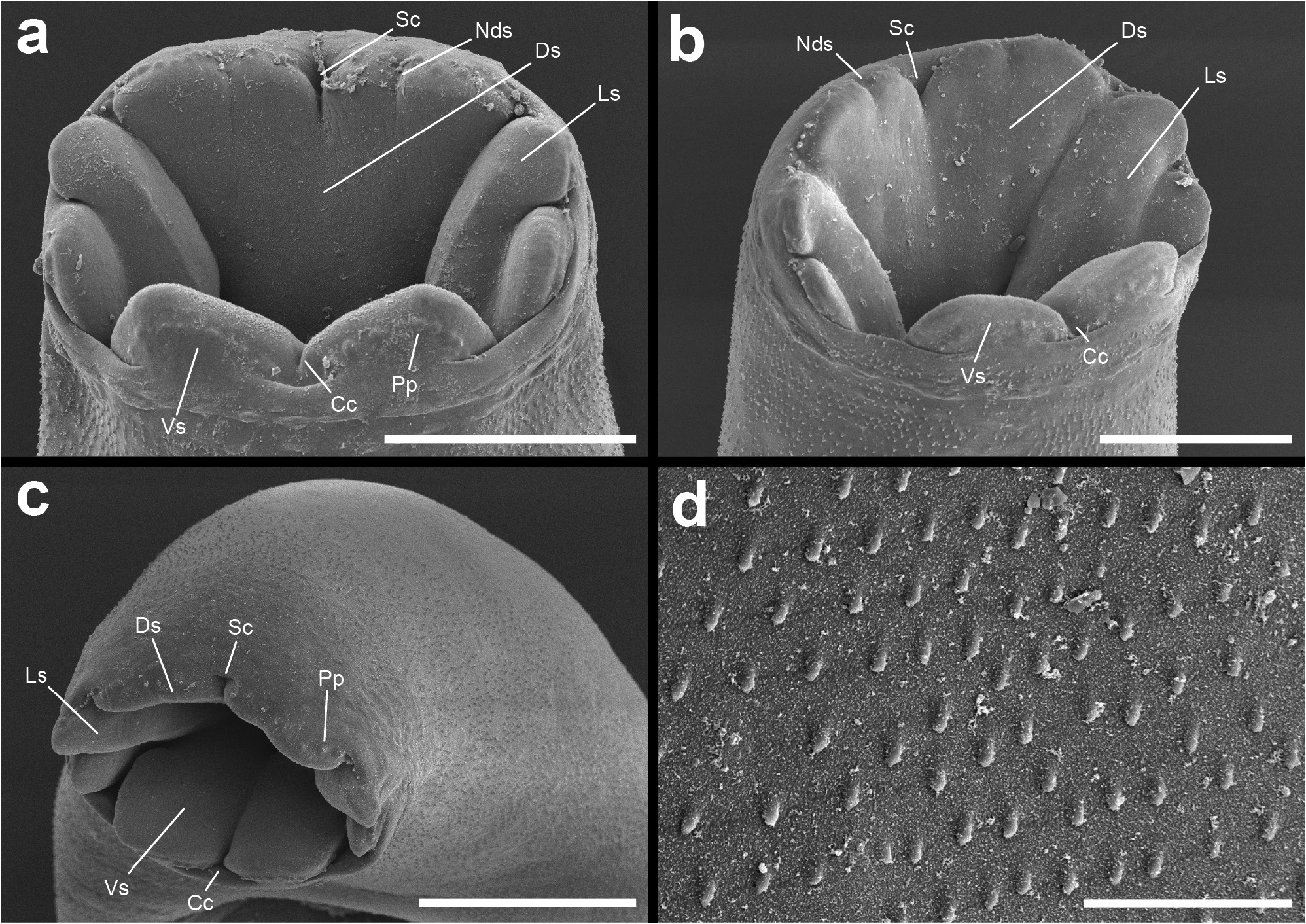
Description: [Measurements in Table 2 View TABLE 2 . Description based on vouchers and SEM images of three adult specimens]. Body robust, elongate, fusiform, slightly dorsoventrally flattened; bright yellow to orange in life with colour fading after preservation in ethanol. Tegument armed with irregular fields of minute, cylindrical spines. Oral sucker terminal, infundibuliform, partially retractable, elaborate, divided into four sections: ventral section comprised of two truncate lobes separated by distinct central cleft and longitudinal crease; lateral sections one either side, each comprised of two oblong lobes separated by distinct notch, with rear, more dorsal, lobe elongate and front, more ventral, lobe shorter and arising from ventrolateral region of rear lobe; dorsal section of oral sucker a single, large lobe with distinct, central, sagittal cleft dividing lobe into two parts, each part with small, central notch. Single row of papillae apparent on outer anterior margin of oral sucker lobes. Ventral sucker robust, round, in anterior third of body, aperture rhomboid. Prepharynx distinct, broad. Pharynx well-developed, ellipsoidal, in mid-forebody. Oesophagus short, narrow. Intestine robust, with well-developed gastrodermis, bifurcates in mid to posterior forebody; caeca reunite in anterior half of post-testicular region to form distinct common stem, attenuates posteriorly, opens at large, dorsally subterminal anus.
Testes two, tandem, separated, ellipsoidal, approximately equal in size, with margins entire to moderately lobed, in posterior half of hindbody, medial, intercaecal. Vasa deferentia separate, narrow, pass more or less parallel along longitudinal median of body anteriorly, enter posterior end of cirrus-sac. Cirrus-sac ellipsoidal, capsule-like, with distinctly thick, muscular walls, intercaecal, extends from just posterior to caecal bifurcation to about midway behind ventral sucker. Internal seminal vesicle tubular, tightly coiled. Pars prostatica indistinct, ellipsoidal, lined with cell-like bodies. Ejaculatory duct muscular, long. Genital atrium narrow. Genital pore opens on single, small, distinct papilla midway between pharynx and anterior margin of ventral sucker.
Ovary subglobular, pre-testicular, intercaecal, medial. Seminal receptacle subglobular, smaller than and postero-dorsal to ovary. Laurer’s canal not observed. Mehlis’ gland profuse, antero-dorsal to ovary. Uterus pre-ovarian, convoluted, intercaecal, passes along ventral margin of cirrus-sac to genital atrium. Eggs numerous, elongate, operculate. Vitellarium follicular; follicles profuse, distributed in dorsal, lateral, and ventral regions of body, extend from posterior margin of ventral sucker to near posterior extremity, wrap around body from dorsal midline to ventrosinistral and ventrodextral regions anterior to testes, wrap around entire body posterior to testes, sparse in testicular region. Vitelline reservoir dorsal to ovary, ellipsoidal to pyriform; collecting ducts indistinct.
Excretory pore dorsally subterminal, posterior to anus. Excretory vesicle tubular, undulates anteriorly along longitudinal midline, passes dorsally to testes, bifurcates in region of ovary; collecting ducts reach as far as oral sucker.
Remarks: Species delineation in the genus Enenterum has relied largely on differences in the number of oral sucker lobes visible by light microscopy of slide-mounted specimens, with counts of two, six, seven, eight, and ten being reported ( Bray & Cribb 2001). However, such counts are likely to miss more subtle structural detail and be heavily influenced by the particular specimen and its method of preservation and preparation. For our specimens of E. kyphosi , the number of oral sucker lobes might be considered as eight or ten, depending upon the specimen and on the interpretation of the smaller notches in the dorsal portion of the oral sucker. These notches are not visible under light microscopy for all specimens. Thus, a count of ten lobes is possible for some specimens but not convincing for others. All other descriptions of E. kyphosi report a count of ten oral sucker lobes ( Yamaguti 1970; Bray 1986; Machida 1993), however, we think there is a strong case to consider our specimens as this species.
Yamaguti (1970) described E. kyphosi based on material from K. cinerascens collected from Hawaii. In addition to being collected from the same host species, our specimens agree well with the original description and have seemingly overlapping morphometrics, although the illustration of E. kyphosi provided by Yamaguti (1970, Fig. 119a) indicate that the holotype was flattened, which impairs morphometric comparison (see Huston et al. 2019a). Flattening may also provide some explanation for the differences in oral sucker lobes, as pressure during fixation may have accentuated the notches in the oral sucker in these specimens. An important feature of E. kyphosi that requires clarification is the accessory structure associated with the genital pore. In our specimens the genital pore opens through a distinct papilla, which we do not consider a true sucker (i.e., a specialised, muscular attachment organ; cf. illustration of the genital sucker of E. petrae n. sp. below). Yamaguti (1970, p. 85) described this in E. kyphosi as a “rudimentary accessory sucker in form of a muscular eversible papillae”. This contrasts with the description of Enenterum elongatum Yamaguti, 1970 , described in the same publication alongside E. kyphosi and from the same host and locality, where the structure was characterised simply as a “rudimentary accessory sucker”. The illustration of the accessory sucker in E. elongatum shares the longitudinal striations typically present in Yamaguti’s illustrations of digenean suckers, but the illustration of the accessory sucker of E. kyphosi does not. Although Yamaguti (1970) may have considered papillae and accessory suckers as homologous structures, we infer that the discrepancy is more likely a result of loose language. Indeed, this appears to be the opinion reached by Bray (1986) in his report of E. kyphosi from South Africa, where he noted the only difference between his specimens and those of Yamaguti (1970) was the “so called accessory sucker, which in these specimens appears to be a genital papilla through which both male and female ducts pass”. Machida (1993) reported E. elongatum and E. kyphosi from Japan and Palau, but unfortunately did not mention accessory suckers or papillae associated with the genital pore in the description of either species or provide illustrations. Machida (1993) also noted difficulties in differentiating these two species and concluded that they may be conspecific, leaving the specific identity of his specimens somewhat ambiguous. The overall morphological evidence does not seem sufficient to consider our specimens as different from the concept of E. kyphosi and, despite the wide geographic spread of reports of this species, we think there is precedent (see Huston et al. 2021b) to consider all reports of E. kyphosi as this species, rather than a complex of morphologically similar species.
Hawaii is a distinct component of the Eastern Indo-Pacific ecoregion ( Spalding et al. 2007) and, because of the exceptional contribution of Yamaguti (1970), has one of the best studied digenean faunas in the IWP ( Cribb et al. 2016). Although several digenean species have been reported from both Hawaii and elsewhere, there is not yet any molecular data confirming conspecificity of Hawaiian forms with those from other ecoregions. However, there is growing genetic evidence that some digenean species which occur in French Polynesia, another component of the Eastern Indo-Pacific, also occur in Australia (e.g., Lo et al. 2001; McNamara et al. 2014; Martin et al. 2018; Huston et al. 2021b; Bray et al. 2022). Furthermore, in a study of the Gorgocephalidae Manter, 1966 , a digenean lineage also restricted to kyphosid fishes, Huston et al. (2021b) provided both morphological and molecular evidence that the range of Gorgocephalus yaaji Bray & Cribb, 2005 , spans the breadth of the IWP, from French Polynesia to South Africa. This species was found to use multiple kyphosids across its range, including K. cinerascens in French Polynesia and K. vaigiensis in South Africa ( Huston et al. 2021b). This distribution provides a precedent for the expectation that the digenean fauna of kyphosids may be widespread and strongly suggests our specimens are conspecific with those reported from Hawaii and South Africa.
Molecular data from Hawaii may show that the forms from elsewhere, including our new material, are actually distinct, but until such data become available, we think considering our specimens as E. kyphosi is the most satisfactory hypothesis.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |