Echinoderes hakaiensis Herranz, Yangel & Leander, 2017
|
publication ID |
https://doi.org/10.5852/ejt.2018.456 |
|
publication LSID |
lsid:zoobank.org:pub:DE1B1DEE-9871-4803-9F67-025F2B439560 |
|
DOI |
https://doi.org/10.5281/zenodo.3818834 |
|
persistent identifier |
https://treatment.plazi.org/id/F64287A2-506A-FFA2-17C0-FB36FBA80B8F |
|
treatment provided by |
Valdenar |
|
scientific name |
Echinoderes hakaiensis Herranz, Yangel & Leander, 2017 |
| status |
|
Echinoderes hakaiensis Herranz, Yangel & Leander, 2017
Material examined
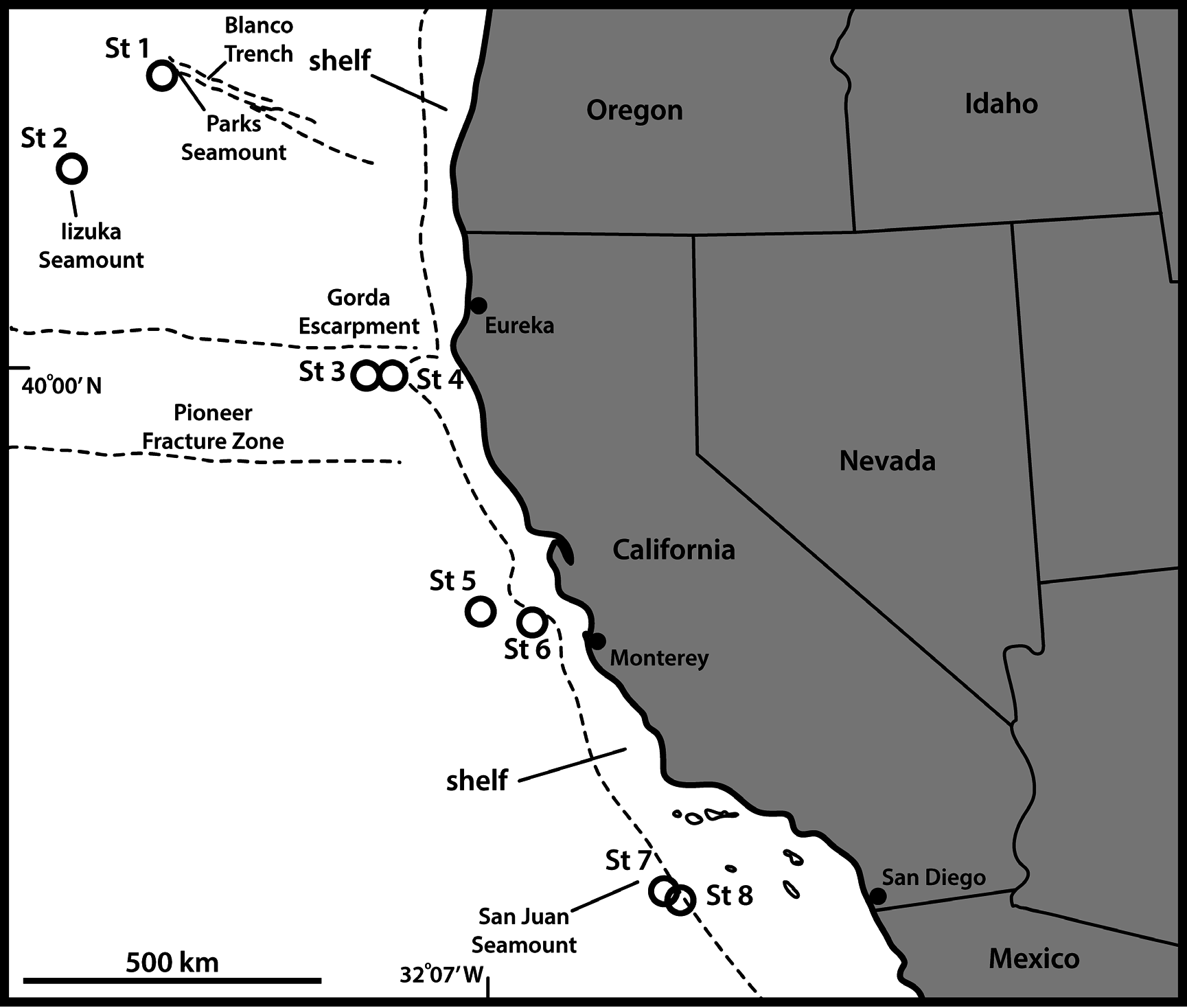
UNITED STATES OF AMERICA: 1 adult ♂, US West Coast, California, off Monterey, 36°40′52″ N, 122°49′37″ W, St. 6, 2719 m deep, 24 Sep. 2008, mounted in Fluoromount G (NHMD-223924); 1 ♂, same collecting data as for preceding, mounted for SEM and stored in the first author’s personal reference collection. See Fig. 1 View Fig for localities and Table 1 View Table 1 for detailed station data. GoogleMaps
Notes on distribution, morphology and comparison with type material
The two recorded specimens show a very close resemblance with E. hakaiensis , a species described from the coast of British Columbia, Canada ( Herranz et al. 2018). The single specimen mounted for LM has a trunk length of 320 µm, which is very close to the mean length of the type specimens ( 324 µm). Moreover, spine and segment lengths are within the range of the type material. The distribution of spines, tubes, sensory spots and glandular cell outlets correspond in detail to the original description given by Herranz et al. (2018), and re-examination of paratypes deposited at NHMD confirmed this compliance. The specimens are not illustrated herein, since no new or different structures were found.
The only considerable difference regards the habitat. Echinoderes hakaiensis was described from a depth of 88–140 m, and is thus a part of the shelf or sublittoral fauna. The recorded specimens originate from the bathyal fauna, which suggests that the species is not limited by depth preferences, but can be found along the American west coast, throughout the sublittoral to bathyal zones.
| US |
University of Stellenbosch |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |