Scipopus ( Scipopus ) erythrocephalus ( Fabricius, 1805 )
|
publication ID |
https://doi.org/10.5852/ejt.2023.904.2323 |
|
publication LSID |
lsid:zoobank.org:pub:C2FCC15D-1DE5-4198-B867-EE4C582BA689 |
|
DOI |
https://doi.org/10.5281/zenodo.10406169 |
|
persistent identifier |
https://treatment.plazi.org/id/F62BA712-1523-FFEA-FE51-F9B71533AD58 |
|
treatment provided by |
Plazi |
|
scientific name |
Scipopus ( Scipopus ) erythrocephalus ( Fabricius, 1805 ) |
| status |
|
Scipopus ( Scipopus) erythrocephalus ( Fabricius, 1805) View in CoL
Calobata erythrocephala Fabricius, 1805: 260 View in CoL
Neria hottentota Robineau-Desvoidy, 1830: 737 View in CoL .
Micropeza loripes Perty, 1833: 188 View in CoL .
Scipopus penicillus Enderlein, 1922: 211 View in CoL .
Scipopus alvarengai Albuquerque, 1972c: 123 View in CoL , figs 1–11. Syn. nov.
Calobata erythrocephala View in CoL – Wiedemann 1830: 532 (diagnosis). — Walker 1849: 1055 (listed). — Osten Sacken 1858: 81 (catalog); 1878: 180 (catalog). — Schiner 1868: 250 (diagnosis). — van der Wulp 1883: 49 (listed); 1897: 367 (keyed), 369 (diagnosis). — Aldrich 1905: 616 (catalog). — Hendel 1922: 231 (listed).
Neria hottentota View in CoL – Enderlein 1922: 210 (synonymized with S. erythrocephalus View in CoL ).
Scipopus penicillus View in CoL – Frey 1927:74 (listed).— Cresson1930:325 (synonymized with S. erythrocephalus View in CoL ). — Curran 1934a: 295, 452 (listed), 451 (keyed). — Schumann 1988: 104 (catalog).
Scipopus erythrocephala View in CoL – Cresson 1930: 325 (diagnosis).
Scipopus erythrocephalus View in CoL – Czerny 1932: 285 (diagnosis). — Hennig 1934: 322 (keyed), 323 (diagnosis). — Aczél 1949: 341 (catalog). — Steyskal 1968: 48.16 (catalog). — Albuquerque 1971: 89, figs 2–11 (re-description). — Ferro & de Carvalho 2014: 59 (listed). — Marshall et al. 2016: 544 View Cited Treatment (catalog).
Micropeza loripes – Hennig 1934: 323 (listed as a synonym of S. erythrocephala ).
Scipopus alvarengai – Ratcliffe & Penny 1978: 695 (catalog).
Differential diagnosis
Scipopus ( Scipopus) erythrocephalus resembles S. ( S.) souzalopesi in having dark tarsomeres and a dull, orange, microtrichose epicephalon that is not clearly delineated from the upper frontal vitta, but it differs in having a black scutum with indistinct silvery vittae.
Type material examined
Holotype ( Calobata erythrocephala ) (damaged thorax and wing on a pin; examined in 2015)
SOUTH AMERICA • “South America”; “Dom. Smidt. Muf. Dom. Lund.”; ZMUC.
Type material ( Neria hottentota )
UNKNOWN • given as “Cap de Bonne-Esperance” in error; type probably lost.
Holotype ( Micropeza loripes )
BRAZIL •?; “Provincia Piauhiensi” [Prov. Plaui]; NMBE.
Paratypes ( Scipopus penicillus )
BOLIVIA • 1 ♂; Prov. Sara; Sep. 1906 – Mar. 1907; 600–700 m a.s.l.; J. Steinbach leg.; MNBG .
BRAZIL • 1 ♀; Pará; 15 Dec. 1893; W.A. Schulz leg.; MNBG .
SURINAME • 1 ♀; Michaelis leg.; MNBG .
Holotype ( Scipopus alvarengai )
BRAZIL • 1?; Pará , Jacaréacanga; Jun. 1969; M. Alvarenga leg.; Malaise; IOC.
Other material examined
BOLIVIA • 1 ♂; La Paz, Heath River Wildlife Centre, ~ 21 km SSW of Puerto Heath ; 12°40′ S, 68°42′ W; 29 Apr.–11 May 2007; CBFC GoogleMaps • 1 ♂, 1 ♀; same collection data as for preceding; 29 Apr.–12 May 2007; S.A. Marshall leg.; DEBU GoogleMaps • 1 ♂; same collection data as for preceding; debu00282040/MYCRO544-19 sequenced for CO1–5′; DEBU GoogleMaps • 2 ♂♂; same collection data as for preceding; S.M. Paiero leg.; CBFC GoogleMaps .
BRAZIL • 1 ♂; Amazonas, Rio Javari, Estirao do Equador ; Oct. 1979; M. Alvarenga leg.; CMNH • 1 ♂, 1 ♀; Mato Grosso, Diamantino, Facienda Sao Joao ; 450 m a.s.l.; 5–6 Feb. 1981; Ekis and Young leg.; CMNH • 1 ♀; same collection data as for preceding; C. Young leg.; CMNH • 1 ♂; Amazonas, Manaus , ZFZ km-11; 2°35′21″ S, 60°6′55″ W; 13 Jan 2020; Rafael and Marshall leg.; INPA (photographed, Fig. 21C View Fig ) GoogleMaps .
COLOMBIA • 1 ♀; Amazonas, Leticia Mocagua ; 150 m a.s.l.; 27 Mar.–3 Apr. 2000; A. Parente leg.; Malaise trap; debu00138373/MYCRO643-20 unsuccessfully sequenced for CO1–5′; DEBU • 2 ♀♀; Amazonas, PNN Amacayacu San Martin ; 3°46′ S, 70°18′ W; 150 m a.s.l.; 12 Mar.–2 Apr. 2001; D. Chota leg.; Malaise; M. 1612; IAVH GoogleMaps • 2 ♀♀; same collection data as for preceding; 2–16 Apr. 2001; M. 1613; IAVH GoogleMaps • 2 ♀♀; same collection data as for preceding; 3°23′ S, 70°6′ W; 15 Oct.–5 Nov. 2001; M. 2767; IAVH GoogleMaps • 1 ♀; Amazonas, PNN Amacayacu Cabaña Lorena ; 3°00′ S, 69°59′ W; 210 m a.s.l.; 1–15 Sep. 2001; J. Parente leg.; Malaise; M. 2202; IAVH GoogleMaps • 1 ♀; same collection data as for preceding; debu01088993/MYCRO542-19 unsuccessfully sequenced for CO1–5′; IAVH GoogleMaps • 1 ♀; Amazonas, PNN Amacayacu, Matamata ; 3°23′ S, 70°6′ W; 150 m a.s.l; 25 Aug.–3 Sep. 2001; D. Chota leg.; Malaise; M.2244; DEBU GoogleMaps • 1 ♀; same collection data as for preceding; 25 Jun.–9 Jul. 2001; M.2031; IAVH GoogleMaps • 1 ♀; same collection data as for preceding; 9–30 Jul. 2001; M.2030; IAVH GoogleMaps • 1 ♀; same as preceding; 3–9 Apr. 2000; A. Felix leg.; IAVH GoogleMaps • 1 ♀; Vichada, PNN Tuparro, Centro Administrativo ; 5°21′ N, 67°51′ W; 100 m a.s.l.; 22 May–3 Jun. 2001; W. Villalba leg.; Malaise; M.1786; IAVH GoogleMaps • 1 ♀; Caqueta Morelia, Rio Bodoquero ; 430 m a.s.l.; 19–20 Jan. 1969; Duckworth and Dietz leg.; USNM .
ECUADOR • 1 ♂, 2 ♀♀; Napo, Jatun Sacha Res. , 6 km E of Misahuali, SOL trail; 1°4′ S, 77°37′ W; 450 m a.s.l.; 30 Apr.–8 May 2002; S.A. Marshall leg.; DEBU (♂ dissected and photographed, Fig. 20D, F View Fig ) GoogleMaps • 1 ♀; same collection data as for preceding; MYCRO823-20/debu179098 sequenced for CO1–5’ (dissected and photographed, Fig. 20B View Fig ); DEBU GoogleMaps • 1 ♀; same collection data as for preceding; varzea; QCAZ (photographed, Fig. 20C View Fig ) GoogleMaps • 1 ♀; same collection data as for preceding; S.M. Paiero leg.; on dung; QCAZ (photographed, Fig. 20A View Fig ) GoogleMaps • 1 ♀; Puerto Orellana, Tiputini Biodiversity Station ; 0°38′2″ S, 76°08′9″ W; Aug. 1999; Kotrba leg.; QCAZ GoogleMaps • 1 ♀; same collection data as for preceding; S.P.L. Luk leg.; QCAZ GoogleMaps • 1 ♂; Napo, Res. ethnica Waorani , 1 km S of Onkone Gare Camp, “Trans.Ent.”; 0°39′10″ S, 076°26′ W; 220 m a.s.l.; T.L. Erwin et al. leg.; insecticidal fogging of mostly bare green leaves, some with covering of lichenous or bryophytic plants in terre firme forest, at Trans 5, stn 7, Project MAXUS, Lot 916; USNM GoogleMaps • 1 ♀; same collection data as for preceding; at Trans 2, stn 5, Lot 864; USNM GoogleMaps • 3 ♀♀; Prov. Orellano, Yasuni Natl. Pk., Yasuni Research Stn ; 0°40′50″ S, 76°24′2″ W; 250 m a.s.l.; 28 Apr.–8 May 2009; S.A. Marshall leg.; QCAZ GoogleMaps • 1 ♀; same collection data as for preceding; debu01015708/ MYCRO540-19 sequenced for CO1–5′; QCAZ GoogleMaps • 1 ♀, 1?; Napo Province, Yasuní National Park, Yasuní Research Station ; 76°36′ W, 0°38′ S; 3–20 Nov. 1998; T. Pape and B. Viklund leg.; QCAZ GoogleMaps .
FRENCH GUIANA • 2 ♀♀; Cayenne , Comm. Regina, Kaw Mt. Relais de Patawam; 4°33′ N, 52°10′ W; 300 m a.s.l.; Dec. 2005; J.A. Cerda leg.; Malaise; DEBU GoogleMaps • 1 ♀; same collection data as for preceding; debu00280292/MYCRO541-19 sequenced for CO1–5′; DEBU GoogleMaps • 1 ♀; Mitaraka ; 2°13′59.8″ N, 54°27′46.5″ W; 471 m a.s.l.; 13–20 Aug. 2015; P.H. Dalens leg.; MIT-E-savane roche 2; MT ( 6 m), MITARAKA/230; MNHN GoogleMaps • 1 ♀; Mitaraka , sites nr base camp and along trails; 1–6 Mar. 2015; J. Touroult and E. Poirier leg.; SLAM, trop. moist forest, MITARAKA/195; MNHN .
PERU • 3 ♂♂, 3 ♀♀; Madre de Dios, Los Amigos Biol. Stn ; 2–14 Jun. 2006; Paiero and Klymko leg.; MUSM • 1 ♀; same collection data as for preceding; debu00281552/MYCRO543-19 sequenced for CO1–5′; DEBU • 1 ♀; Madre de Dios, CICRA, Trail GoogleMaps 2; 12.56104° W, 70.10645° S; 267 m a.s.l.; 1–6 Jan. 2014; T. Perez leg.; MHNJP • 2 ♀♀; Cusco, Est. Biol. Villa Carmen ; 12°54′ S, 71°24′ W; 500–700 m a.s.l.; 22 Jun. 2014; trap VC-ML-84; MHNJP GoogleMaps • 1 ♀; same collection data as for preceding; 31 May 2014; trap VC-ML-80; MHNJP GoogleMaps • 1 ♀; same collection data as for preceding; 520–580 m a.s.l.; 8 May 2014; M. Choque leg.; multi-lure traps; USNM GoogleMaps • 1 ♂; same collection data as for preceding; S side; 527 m a.s.l.; Jan 2014; Malaise trap; USNM GoogleMaps • 1 ♀; Loreto Yanamono GoogleMaps ; 3°26.520′ W, 72°50.9′ S; 14 Mar. 2004; W. Reeves leg.; MUSM .
VENEZUELA • 1 ♂; Sucre, El Rincón, nr road between Carúpano and El Piler, along Rio El Rincón valley ; 10°35′56″ N, 63°11′49″ W; 84 m a.s.l.; 8–9 Apr. 1998; M. von Tschirnhaus; 1° and 2° forest, sweep, river bank vegetation; debu00256946/MYCRO567-19 sequenced for CO1–5′; DEBU GoogleMaps .
Description
LENGTH. 13–16 mm.
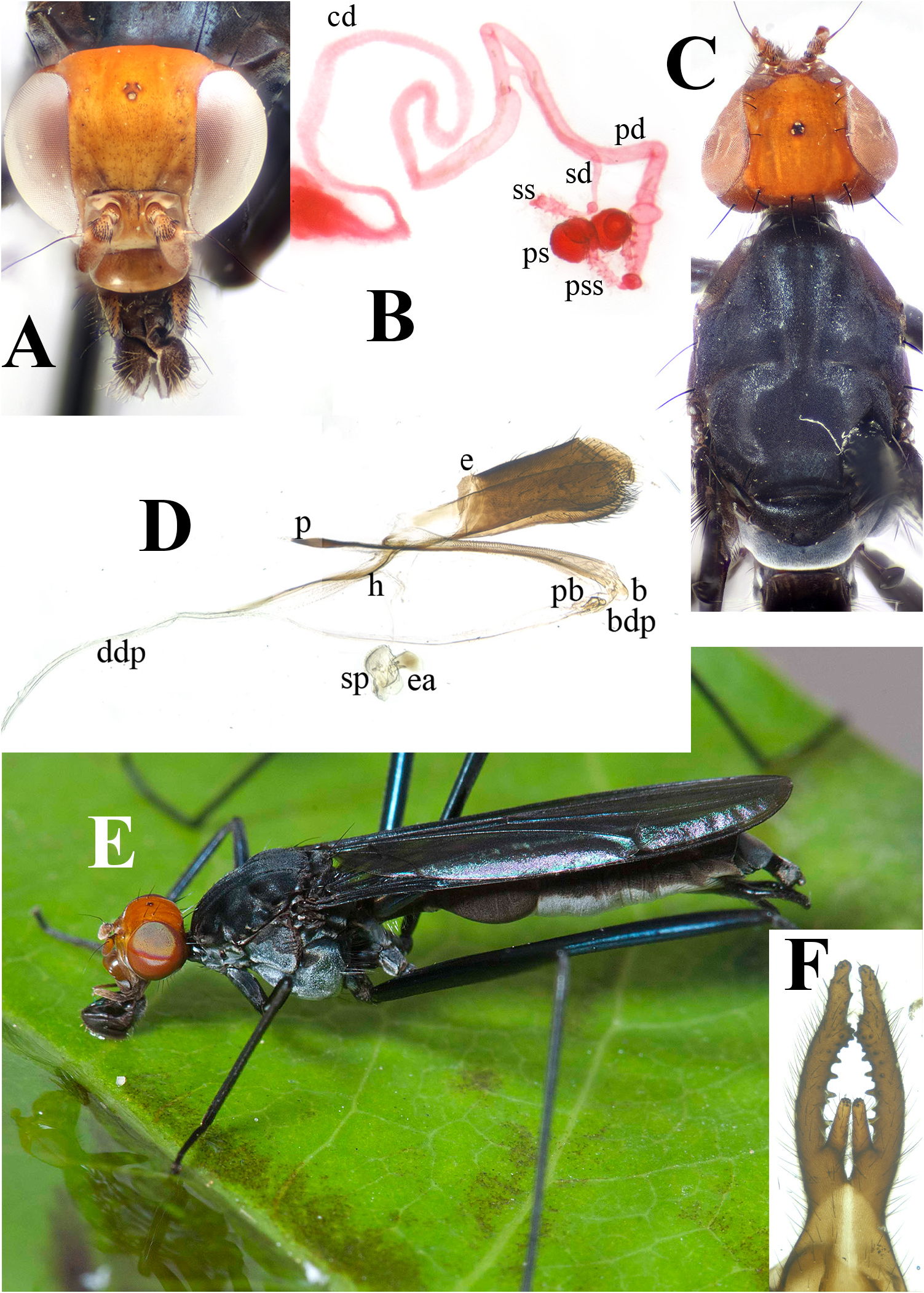
HEAD. Palpus orange, brown basally, pale brown microtrichose and setulose on entire surface, denser on ventral edge and apex, narrow (length 5.5× height). Clypeus orange or light brown, shiny, width ~2.0× height, bare medially, white microtrichose in posterolateral corners. Frontal vitta dull, orange, microtrichose. Orbital plate orange, microtrichose, with shiny anterior orange or light brown bare patches ( Fig. 20C View Fig ). Epicephalon dull, orange, microtrichose, not clearly delineated from upper frontal vitta. Paracephalon orange or light brown, slightly convex on posterolateral portions. All head chaetotaxy well-developed.
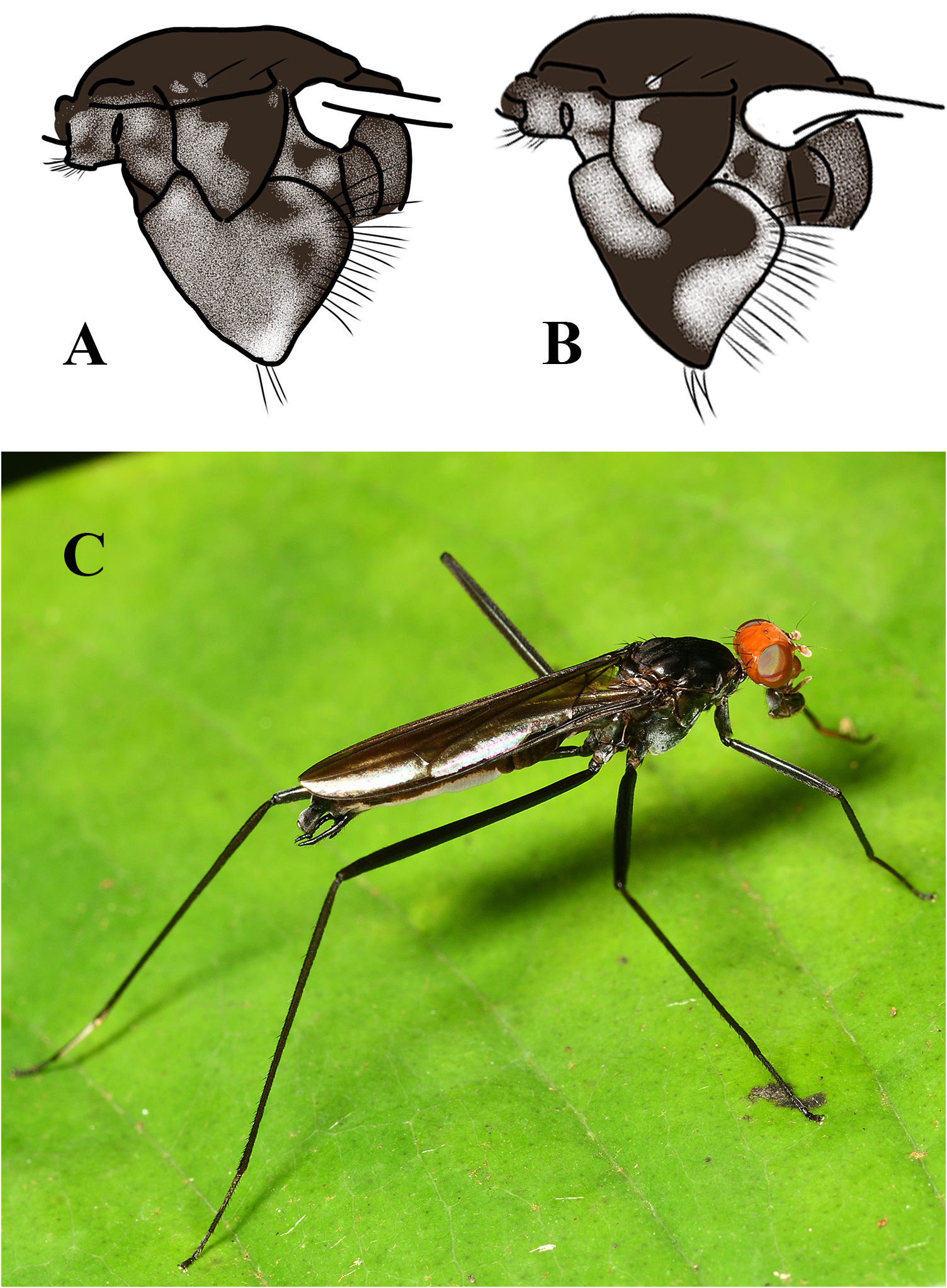
THORAX. Scutum black, with sparse silvery microtrichosity and three indistinct silvery anteromedian vittae. Female cervical sclerite slightly convex anteriorly. Postpronotal lobe black or brown, with very few setulae on anterior outer margin. Notopleuron black with 1–2 small pale microtrichose spots. Pleuron with the following microtrichosity: entirely white on proepisternum, white except brown median line on proepimeron, white on anteroventral ½ of anepisternum, otherwise brown, white with brown median spot on katatergite. Microtrichosity on katepisternum ranging from entirely white to mostly white, with brown ventral and dorsal patches or spots ( Fig. 21A View Fig ), to white anteriorly and posteriorly, with remainder brown ( Fig. 21B View Fig ).
ABDOMEN (J+ ♀). T1 with fine, long, white setae.
FEMALE ABDOMEN. Pleuron pale grey, microtrichose; P1 and dorsal half of P2–6 dark brown. T1+2 ~2.0× length of T3. Oviscape black, white microtrichose on anteroventral ½, ~2.0–3.0× length of T6. Combined spermathecal ducts long; common duct long, ⅔ to ¾ of entire duct length. Paired spermathecal duct narrow, short (5.0–7.0× length of paired spermathecae), parallel-sided, distally smooth with swollen bulb ( Fig. 20B View Fig ), or with distal striae. Paired spermathecal stems same length or longer than spermathecae, relatively inornate but with some small tubercles. Paired spermathecae narrower basally, with well-defined groove or bend on basal ⅓, separating spermathecae into smaller basal portion and larger distal portion. Single spermathecal duct arising from basal ¹/10 of paired duct, narrow, ½ the diameter and ≈ length as paired duct, swollen apically. Single spermatheca elongate, finger-like, with minute spiked tubercles.
MALE ABDOMEN. Pleuron pale grey, microtrichose, dark grey or brown on P1, most of P2 and dorsal half of P3–6 ( Fig. 20E View Fig ). T1+2 ~2.0 × length of T3. Genital fork large (3.0× length of T6), distal ends straight or converging, inner basal process pointed inward or straight, ~⅓–½ length of arm. Epandrium elongate, length 2.0× height, densely setose on posteroventral margin. Basiphallus small, projecting posteriorly, crescent-shaped. Basal distiphallus very short (shorter than phallic bulb), ending in phallic bulb. Phallic bulb short and small, length ≈ height, with upper and lower chamber and rounded posterior projection. Distal distiphallus very long,>3.0× length of epandrium. Phallapodeme slender, slightly expanded distally.
Remarks
The type of this species is severely damaged and only one wing remains; however, the original descriptions and treatments of the species by Hennig (1934) and Albuquerque (1971) are consistent with our concept of the species. Scipopus alvarengai is synonymized with S. ( S.) erythrocephalus based on the similar pleural patterns on the thorax, the similar male and female terminalia (including the exceptionally large genital fork), and molecular data.
Distribution
Steyskal (1968) gives the distribution of this species as Panama south to Brazil and Bolivia, but the material we have examined suggests that S. ( S.) erythrocephalus is restricted to South America.
| ZMUC |
Zoological Museum, University of Copenhagen |
| NMBE |
Naturhistorisches Museum der Burgergemeinde Bern |
| IOC |
Colecao de Culturas de Fungos do Instituto Oswaldo Cruz |
| DEBU |
Ontario Insect Collection, University of Guelph |
| CMNH |
The Cleveland Museum of Natural History |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
| IAVH |
Instituto de Ivestigacion de los Recursos Biologicos Alexander von Humboldt |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| QCAZ |
Museo de Zoologia, Pontificia Universidad Catolica del Ecuador |
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Nerioidea |
|
Family |
|
|
SubFamily |
Taeniapterinae |
|
Genus |
Scipopus ( Scipopus ) erythrocephalus ( Fabricius, 1805 )
| Lindsay, Kate & Marshall, Stephen A. 2023 |
Scipopus alvarengai
| Albuquerque L. P. 1972: 123 |
Scipopus erythrocephalus
| Marshall et al. 2016: 544 |
| Ferro G. B. & de Carvalho C. J. B. 2014: 59 |
| Albuquerque L. P. 1971: 89 |
| Steyskal G. C. 1968: 56 |
| Aczel M. L. 1949: 341 |
| Hennig W. 1934: 322 |
| Hennig W. 1934: 323 |
| Czerny L. 1932: 285 |
Scipopus penicillus
| Schumann H. 1988: 104 |
| Curran C. H. 1934: 295 |
| Curran C. H. 1934: 452 |
| Curran C. H. 1934: 451 |
| Cresson E. T. 1930: 325 |
| Frey R. 1927: 74 |
Scipopus penicillus
| Enderlein G. 1922: 211 |
Neria hottentota
| Enderlein G. 1922: 210 |
Micropeza loripes
| Perty M. 1833: 188 |
Neria hottentota
| Robineau-Desvoidy J. B. 1830: 737 |
Calobata erythrocephala
| Hendel F. 1922: 231 |
| Aldrich J. M. 1905: 616 |
| van der Wulp F. M. 1897: 367 |
| van der Wulp F. M. 1897: 369 |
| van der Wulp F. M. 1883: 49 |
| Osten Sacken C. R. 1878: 180 |
| Schiner J. R. 1868: 250 |
| Osten Sacken C. R. 1858: 81 |
| Walker F. 1849: 1055 |
| Wiedemann C. R. W. 1830: 532 |
Calobata erythrocephala
| Fabricius J. C. 1805: 260 |