Metarctia (Hebena) smithi Fiebig, László, Volynkin & Taberer, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5339.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:66C2F960-8635-4642-84CA-FEDF371B266F |
|
DOI |
https://doi.org/10.5281/zenodo.8309344 |
|
persistent identifier |
https://treatment.plazi.org/id/DE70A656-C85A-470A-935C-1215424BE7CA |
|
taxon LSID |
lsid:zoobank.org:act:DE70A656-C85A-470A-935C-1215424BE7CA |
|
treatment provided by |
Plazi |
|
scientific name |
Metarctia (Hebena) smithi Fiebig, László, Volynkin & Taberer |
| status |
sp. nov. |
Metarctia (Hebena) smithi Fiebig, László, Volynkin & Taberer View in CoL sp. n.
(Figs adults 7a–e, 19, 29; genitalia 46a–b, 56; habitats 83–84)
Holotype. Male , “ ZAMBIA 1340m Jiwundu Swamp S11°51’54”, E25°33’20” 21-24.xi.[20]14 Light Trap leg. Smith, R. & Takano, H. ANHRT:2017.12”, unique id.: ANHRTUK 00010165, DNA barcode id.: ANHRTUK00010165/BC ZSM Lep 113348, BOLD process id.: GWOUH907-21, gen. slide No.: AV 4368 ( ANHRT).
Paratypes (75 males, 8 females). Zambia. 4 males, 1 female, with the same data as in the holotype, gen. slide Nos: AV 4376, AV 4383, AV 4389 (males), AV 4404 (female); 1 male, 1 female, Kambishi School, 1346m, 11°54’42”S, 25°28’50”E, 10–13.xi.2017, MV Light Trap, Carter, M., Lloyd, A., Miles, W., Oram, D., Smith, R. leg., ANHRT:2017.32; 4 males, Zambezi Rapids (Miombo/Riverine forest mosaic), 1205m, 11°7’30”S, 24°11’6”E, 4–9.xi.2018, MV and Actinic Light Trap, Aristophanous, M., Dérozier, V., László, G., Oram, D. leg., ANHRT:2018.40; 5 males, Chitunta Plain (Miombo/ Dambo mosaic), 1396m, 11°29’12”S, 24°24’18”E, 29.xi.–4.xii.2019, MV and Actinic Light Trap, Bashford, M., Miles, W., Mulvaney, L. leg., ANHRT:2019.25, DNA barcode id.: ANHRTUK00125338/BC ZSM Lep 113324, BOLD process id.: GWOUH883-21; 2 males, Nyangombe Falls, (Miombo/Riverine forest mosaic), 1300m, 11°48’25”S, 24°32’12”E, 15– 17.xi.2018, MV Light Trap, Aristophanous, M., Dérozier, V., László, G., Oram, D. leg., ANHRT:2018.40, DNA barcode id.: ANHRTUK00073799/BC ZSM Lep 113329, BOLD process id.: GWOUH888-21, gen. slide No.: AV 6408; 2 males, 1 female, Mwinilunga, Nkwaji, 1316m, 11°36’22”S, 24°33’17”E, 3–10.xi.2017, MV Light Trap, leg. Carter, M., Lloyd, A., Miles, W., Oram, D., Smith, R. leg., ANHRT:2017.32; 15 males, Hillwood, Ikelenge (Miombo/Riverine forest mosaic), 1400m, 11°16’02”S, 24°18’59”E, 23–30.xi.2019, MV, Actinic and LepiLED Light Trap, Bashford, M., Miles, W., Mulvaney, L., Smith, R. leg., ANHRT:2019.25, DNA barcode id.: ANHRTUK00122370/BC ZSM Lep 113339, BOLD process id.: GWOUH898-21; 2 males, same site, but collected at 21–28.x.2013, Smith, R., Takano, H., Chmurova, L., Smith, L. leg.; 1 male, same site, but collected at 17–24.iii.2013, Smith, R., Takano, H. leg., gen. slide No.: AV 4373; 17 males, Kapishya Hot Springs, Shiwa N’gandu Estate, 1437m, 11°10’13”S, 31°36’00”, 14–16.iii.2017, M.T. Harvey coll., Oram, D., Miles, W., Smith, L. leg., ANHRT:2017.30; 4 males, 2 females, same site and collectors, but collected at i.–iii.2017, ANHRT:2017.30, DNA barcode id.: ANHRTUK00048732/BC ZSM Lep 113358, BOLD process id.: GWOUH917-21, gen. slide No.: AV 6405 (female); 4 males, same site, but collected at i.–iii.2016, M.T. Harvey coll., Smith, R., Takano, H. leg., ANHRT:2017.29; 2 males, same site, but collected at 1–5.iii.2017, Oram, D., Miles,W.Smith, L. leg., ANHRT:2017.24, DNA barcode id.: ANHRTUK00160167/BC ZSM Lep 113327, BOLD process id.: GWOUH886-21; 3 males, 1 female, same site, but collected at xii.2014, M.T. Harvey coll., Smith, R., Takano, H. leg., gen. slide No.: AV 4366, AV 4371, AV 4384 (males), AV 4405 (female); 1 male, 1 female, Lumangwe Falls, Kalungwishi River, 1187m, 09°32’33”S, 29°23’17”E, 20–22.xi.2012, Light Trap, Smith, R., Takano, H. leg., ANHRT:2017.7, DNA barcode id.: ANHRTUK00194657/BC ZSM Lep 113337, BOLD process id.: GWOUH896-21, gen.slide No.:AV 4390 (male), DNA barcode id.:ANHRTUK00194658/ BC ZSM Lep 113350, BOLD process id.: GWOUH909-21, gen. slide No.: AV 4403 (female); 2 males, Camp near Kanyama, (Miombo/Riverine/Dambo mosaic), 1375m, 11°25’36”S, 24°40’00”E, 4–7.xii.2019, Actinic Light Trap, Bashford, M., Miles, W., Mulvaney, L. leg., ANHRT:2019.25; 1 male, Kasanka River Pontoon, Kasanka N.P., 1191m, 12°34’23”S, 30°14’05”E, 2–4.xii.2012, Light Trap, Smith, R. Takano, H., leg., gen. slide No.: AV 4374; 1 male, Kabwe, Kasanka N. P., 1187m, 12°32’28”S, 30°12’42”, 14–17.v.2013, Light Trap, Smith, R., Takano, H., Oram, D. leg., gen. slide No.: AV 4387; 1 male, Lukulu River, Lavushi Manda N. P., 1285m, 12°15’05”S, 30°53’43”E, 27–29.xi.2012, Light Trap, Smith, R. Takano, H. leg., gen. slide No.: AV 4388 ( ANHRT); 1 male, Mayukuyuku, Kafue NP, S 14°54’55”S 26°03’47”E, 1080m, 21.–26.xi.2013, leg. Smith, R., Takano, H., DNA barcode id.: BC ZSM Lep 111914, BOLD process id.: GWOUH518-21, gen. slide No.: RF 565.2020 ( RCMS); 1 male, 1 female, NW, 50 km E of Mwinilunga, 28.x.2008, leg. Snizhek, gen. slide No.: GP 26.891, GP 32.195 (female) ( MWW).
Zimbabwe. 1 male, Manicaland, Upper Bvumba , 1675m, 19°07.238’S, 32°46.171’E, 24.ii.2017, A.J. Kingston leg., gen. slide No.: LG 5633 ( RCAK) GoogleMaps .
Diagnosis. Metarctia smithi sp. n. is hardly distinguishable from other Hebena taxa displaying red-highlighted venation. It is worth noting, however, that M. smithi (together with M. haraldsulaki ) does not seem to have the pale monochromatic form which is prevalent in M. rubra , M. manfredi and the M. henrardi-lukaszi species-pair. The wing pattern in the taxa of the M. smithi lineage and M. lateritia does not provide adequate characters for identification. There are, however, reliable diagnostic features expressed in the shape of the antenna and the colouration of the labial palps and legs. The species of the M. smithi lineage have somewhat shorter antenna with slightly longer rami, compared to M. lateritia . The labial palp is black dorsally in M. smithi , whereas it is entirely brick-red or dorsally yellowish in M. lateritia ; additionally, the tibia of the mid- and hindlegs are mostly uniformly dark grey, sometimes with an admixture of sparse orange-red hairs in the M. smithi lineage, while it is fully covered by reddish hairs dorsally in M. lateritia . In the male genitalia, M. smithi is clearly distinguished from the other taxa of the subgenus by a set of characters such as the modified uncus bearing a baso-dorsal bulge, the presence of lateral lobes of the tegumen, the lack of the dorsal protrusion of the valva costa and the apically bifurcate terminal process of the valva in contrast to the simple valva tips of the other lineages of the subgenus (except for M. manfredi which also has dilated valva apex). In the female genitalia, M. smithi is characterized by the presence of a distal scobinate-rugose plate of the eighth sternite bearing a pair of small, semi-spherical sacks, and the presence of a thick, heavily sclerotized, ribbon-like lamella postvaginalis which characters are absent in other Hebena species.
Description. Forewing length 17–22 mm in males, 21–24 mm in females. Male antenna bipectinate-ciliate, black, rami ca. 5 times longer than diameter of shaft, cilia very short, white. Female antenna serrate, rather thick, black. Head. Small, proboscis small, labial palp short, brick-red ventrally, tip and dorsal side black. Compound eye small, spherical, frons and vertex brick-red. Thorax. Collar and tegula brick-red with admixture of greyish hair-scales; mesothorax brick-red. Legs. Coxa and femur covered in thick brick-red to orange-red hairs, foreleg tibia with orange-red hairs, mid- and hindleg tibia and tarsus entirely black; index of spurs: 0-2-2. Forewing. Moderately broad, elongate, especially in female, dorsal margin almost straight, subapically slightly convex, apex rounded, outer margin long, evenly arcuate, anal margin short, almost straight. Ground colour graphite-grey to brownish-grey; veins covered in salmon to crimson scales; transverse lines absent; fringe short, monochromatic ochreous to pinkish-orange. Underside. Similar to upperside with more extensive reddish suffusion in basal half and less contrasting veins. Hindwing. Well-developed, short and narrow, apically rounded, uniformly ochreous to salmon without marking, in females often with slightly darker dorsal margin; fringe short, colour as of hindwing. Underside as upperside. Abdomen. Stout, orange-red, ringed with black proximally.
Male genitalia. Uncus short, basally with a swollen dorsal bulge, medially gently curved, apically pointed with apex directing ventrad. Uncus base dilated with rounded, dentate margin. Tegumen short with well-developed triangular lateral lobes or rounded lateral protrusion with dentate margin, arms fused close to uncus base. Valva very broad at base, transtilla short, costal margin without projection and setae; valva costa directly continued in short, distally dilated, apically bifid terminal process of valva bearing a short, rounded ventral process and a longer, pointed dorsal process. Sacculus short and narrow, distally with a variably setose gently arcuate crest. Anellus (fultura superior) large, more or less quadrangular, fused with inverse Y-shaped juxta (fultura inferior) of variable width. Vinculum moderately long, distal section produced, apex rounded. Phallus moderately long and thin bearing a longitudinal belt of small spinules, medially slightly flexed, coecum very short, rounded, apex without carina process. Vesica with slightly dilated proximal section bearing a small, densely spinulose subbasal field dorsally, and an extensive spinulose area ventrally consisting of extremely small, sparse spinules; distal section of vesica narrow, tubular.
Female genitalia. Papilla analis short, rounded-trapezoidal, barely setose; posterior apophysis moderately long (ca. as long as papilla analis), thin, pointed. Eighth tergite very short, evenly arched, ribbon-like; anterior apophysis almost fully reduced. Eighth sternite with a broad V-shaped, finely scobinate, rugose plate distally and a pair of finely scobinate, short sack-like projections proximally. Ostium bursae narrow with moderately sclerotized ovoid margin. Lamella postvaginalis present, thick and heavily sclerotized, fully fused with amorphous, rugose lamella antevaginalis. Ductus bursae strongly sclerotized, short, relatively thick, tubular; cervix bursae unmodified. Tubular distal part of corpus bursae membranous, very short, one-third as long as ductus bursae; dilated proximal section of corpus bursae large, spherical; signum bursae represented by a long (ca. half as long as bursa copulatrix) and narrow, strongly sclerotized, scobinate field.
Genetic information. Metarctia smithi is a genetically rather heterogeneous taxon assigned to four different BIN URIs: BOLD:AEM0979, AEL9858, AEM2045, and AEM2044. However, the distinct BINs do not coincide with any constant diagnostic morphology or allopatric distribution, and hence all four genetic clusters are considered to represent only genetic variations within the species with high intraspecific divergence in the range of 0.33 and 4.92%. The nearest neighbour of M. smithi is its southwest Tanzanian subspecies M. smithi transvallesiana , diverging at 3.14−4.50%. The pairwise distance between the nominotypical M. smithi and M. haraldsulaki is 3.50−5.31%. Compared to the topotypical population of M. lateritia (BIN URI: BOLD:AAZ9706), M. rubra , M. elleni and M. brigitta , the pairwise genetic distances are 5.81−8.74%, 5.24−6.80%, 5.21−7.61% and 5.56−6.62%, respectively.
Etymology. This new species is dedicated to Richard Smith, founder and Chairman of the Board of Trustees of the African Natural History Research Trust, prominent supporter of entomological research in Sub-Saharan Africa and one of the collectors of the new species.
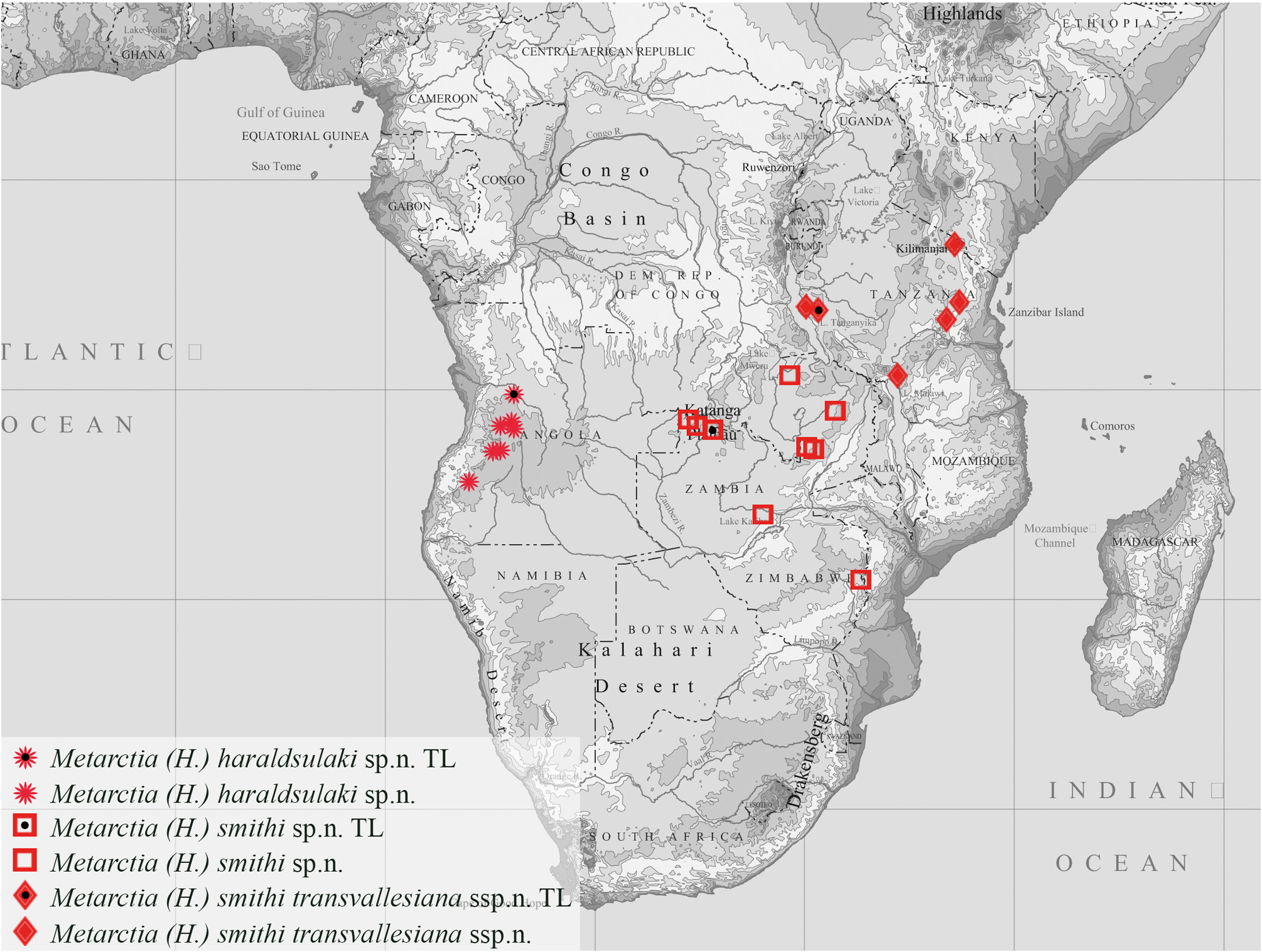
Distribution ( Map 3 View MAP 3 ). Metarctia smithi is known to date from the Zambian Plateau and the Bvumba Mountains.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Arctiinae |
|
Genus |