Psilorhynchus tenura, Arunachalam & Muralidharan, 2008
|
publication ID |
https://doi.org/10.5281/zenodo.5340876 |
|
persistent identifier |
https://treatment.plazi.org/id/E85B87A1-FFA3-FFFC-4BCD-D0C0F3AC6C75 |
|
treatment provided by |
Diego |
|
scientific name |
Psilorhynchus tenura |
| status |
sp. nov. |
Psilorhynchus tenura View in CoL , new species
( Fig. 1 View Fig )
Material examined. – Holotype: ZSI/SRS F.7600, 51.9 mm SL, Male; India: Karnataka, Korkanhalla, Tributary of Thunga River inside the Khudremukh National Park , ( 13°20'22.3"N 75°10'19.4"E); colls. M. Arunachalam & P. Sivakumar, 23 Feb.2002. GoogleMaps
Paratypes: MSUMNH 45, 3 ex, 46.4–50.8 mm SL; CMA12, 2 ex, 41.6–44.6 mm SL; same locality data as holotype.
Diagnosis. – Lateral-line scales 35–37 (33–35 up to hypural base with 2–3 scales on caudal fin), pectoral fin with 5 or 6 simple and 10–12 branched rays, caudal-fin rays 17 (9 + 8), body cicumferential scales 15, body shallow 16.8–19.8% SL, slender and short peduncle (depth 5.6–7.1% SL and length 13.7–16.2% SL). Ventral region free from scales, however 3–4 scales rows found between pelvic fin insertion and pectoral-fin insertion along the edge of the body. Eye in the upper part of the head, barely visible from ventral aspect. Seven dorsal spots, three predorsal and four postdorsal. Eight spots in the body placed in the space between the dorsal spots along the lateral line. Paired fins with a spot at the insertion region, and the membrane between simple ray and few anterior branched rays black.
Description. – Body shape and appearance as shown in Fig.1 View Fig . The morphometric data of all the types are provided in Table 1. Head depressed, mouth transverse inferior. Upper lip separated from upper jaw by a deep groove and from snout by a shallow groove. Deep grooves on both sides separate ventral surface of snout from lateral surface. Upper lip joined to lower lip at corner of mouth by a prominent flap of skin. Lower lip thick with the presence of papillae distinct on the anterior region gradually decreasing towards posterior end. Mandible length greater than the gape width. Gill aperture extends dorsally upwards from slightly anterior to pectoral fin base from the ventral part of head. Eye large, placed dorsolaterally; upper rim of orbit on level with interorbital. Interorbital region flat to slightly concave. Orbit diameter equal to (or) slightly greater than interorbital width. Head conical with the tip slightly blunt in ventral aspect.
Predorsal scales 11–12, 4 scales in transverse series above lateral line with mid dorsal scale row but excluding scale along lateral line and 3 scales below with the lateral line but excluding the small scale row at the pelvic base, circumferential scales 15, circumpeduncular scales 10, scales between anus and anal fin 11–12. Paired fins inserted horizontally. Pectoral with 5–6 simple and 10–12 branched rays, its tip extending vertically beneath dorsal fin origin when compressed, leaving 2 scale rows before pelvic fin insertion. Pelvic fin with two simple and seven branched rays. Pelvic fin origin about 2 scale-widths posterior to dorsal fin origin. Dorsal fin with 3 simple ( 2 in 1 ex.) and 8 branched ( 9 in 1 ex.) rays (last branched ray not split at base and is counted as one). Posterior part (last branched ray) of the dorsal fin longer than anterior (unbranched ray) in depressed dorsal fin, with distal margin concave when it is expanded. Anal fin short and falcate (tip curved). Pointed edge of anal fin not reaching caudal fin. Anal fin with 3 simple and 5 branched rays. Caudal fin deeply forked, with equal caudal lobes with 17 (9/8) principal rays, about 6 or 7 procurrent rays in the upper lobe and 4 or 5 in the lower lobe of the fin.
Tubercles. – Tubercles distinct on males, distributed on head rather not uniformly. Region below the eye to lower part of cheek with the presence of closely packed tubercles. Tubercles also found on the scales in the anterior part of body mostly on the free margins. Posterior region of the body with fewer tubercles. Males with row of tubercles on the dorsal surface of pectoral fins rays. Tubercles gradually reducing in size towards posterior part of pectoral fin. Females with fewer tubercles not as evident as in males, further they lack tubercles in the paired fins.
Colouration. – Eight or nine dark lateral blotches of variable size (two to three scales wide and one to two scales high) and shape (round to oblong) with the last blotch placed on caudal base that extends a little to caudal fin. Anterior-most blotch on lateral line immediately posterior to gill cover. A row of seven dark spots on the dorsum, three in the predorsal region and four behind dorsal base. Spots progressively reduced in size towards tail, the posterior ones the smallest. Spots on the dorsum neither coalesce nor come in vertical line with transverse blotches with an exception of the last transverse blotch, which coalesces with the dorsal spot at the caudal base. Scales with dark margins. Dorsal region of head dark. Two dark bands, one from each nostril extend forward to meet anteriorly at tip of snout. Caudal fin with a faint band along the principal ray and first two branched rays on both upper and lower lobes, joining the blotch at the caudal base. Dorsal fin with similar small scattered melanophores on the simple rays. Paired fins with darkened membranes between third and fourth branched rays.
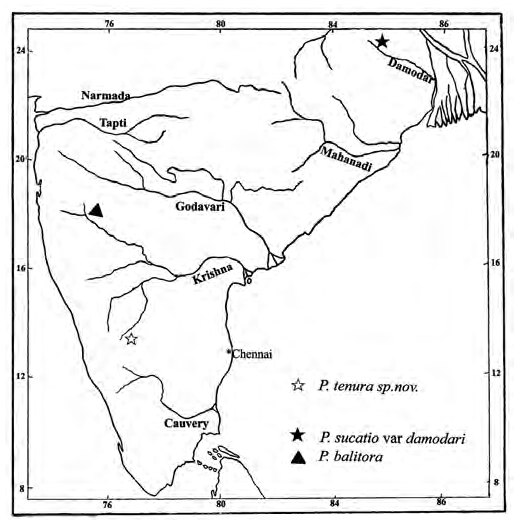
Distribution. – At present known only from Korkanhalla stream ( Fig. 3 View Fig , type locality). It prefers cobbled substrate, which shows the possibility of its occurrence in all other similar streams with more cobbles in Thunga and Bhadra rivers.
Etymology. – The name tenura derived from Latin tenuis meaning thin, slender and Greek oura meaning tail, in reference to the slender peduncle region. A noun in apposition.
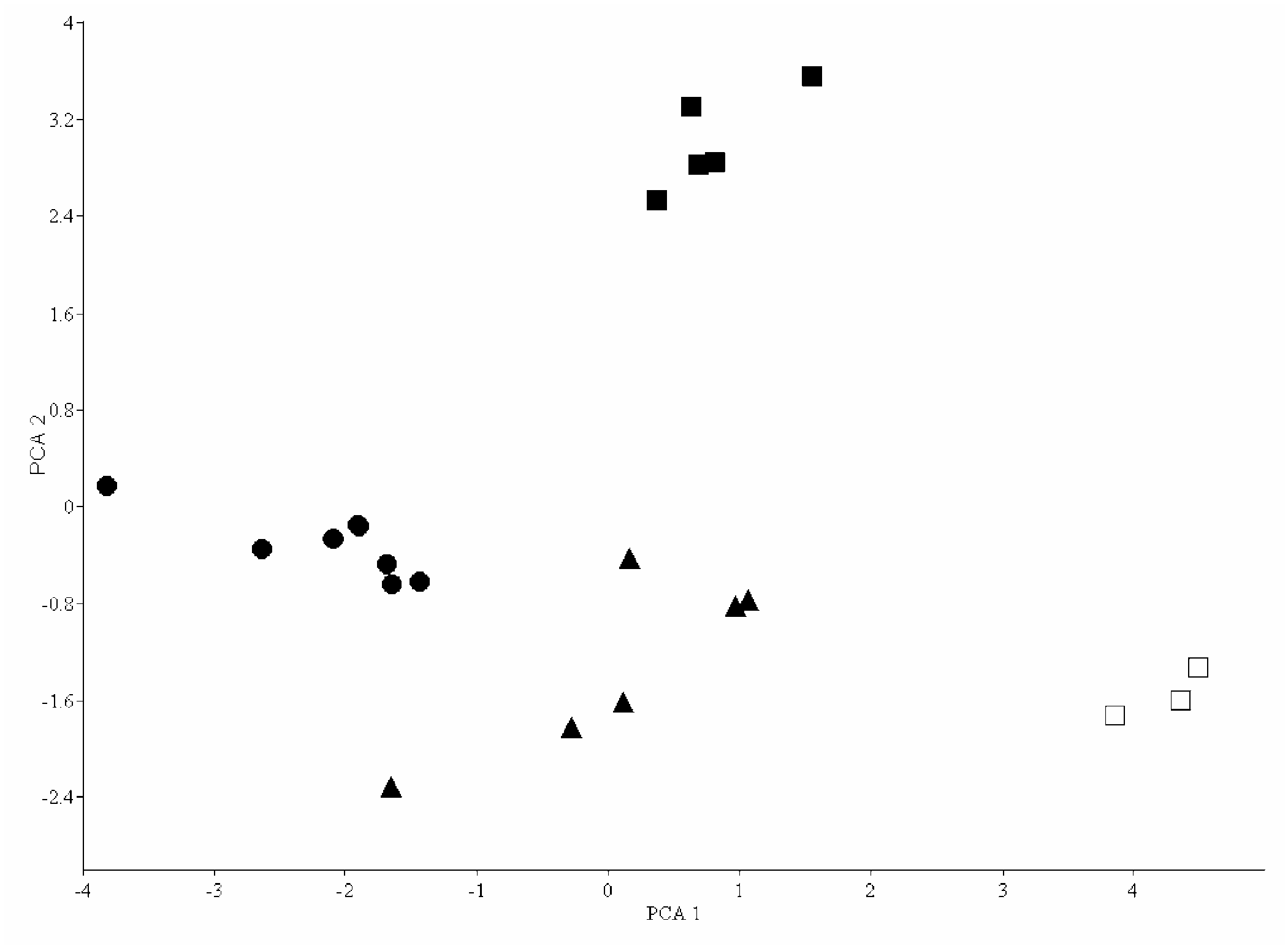
Comparison of species. – A comparative study on the morphometric and meristic variables though show resemblances with the congeners, there is a set of perfectly discriminating characters that distinguish the new species from the related species ( Tables 2 & 3). The new species P. tenura closely resembles P. gracilis but differs in caudal rays [17 (9 + 8) vs. 19 (10 + 9)], predorsal scales (11–12 vs. 10), circumferential scales (15 vs. 16–17) and anal scale rows between anus and anal fin origin (11–12 vs. 9–10). It also shows variation in morphometric characters such as body depth (16.8 – 19.8 vs. 21.5 – 23.7% SL) peduncle depth (5.6–7.1 vs. 8.8 –9.5% SL), peduncle length (13.7–16.2 vs. 15.5–19.1% SL), gape width (25.6–29.4 vs. 19.2–22.3% HL) and mandible length (26.1–32.7 vs. 31.5–35.7% HL). Among these, body depth, peduncle depth and length, gape width demonstrate a considerable proportion of variation to show a more slender body, short and slender peduncle and wide gape width in P. tenura . Colouration is similar in both the species and the total number of dorsal blotches is in the same range; the number of blotches in predorsal and post-dorsal region, however, varies. Another related species P. sucatio though similar in lateral-line scale count has a scaled belly, while the abdominal region is naked in P. tenura . Meristic variations are also evident, including the number of simple rays in dorsal, branched rays in dorsal and the branched rays in pectoral. The number of circumferential scales 15 in P. tenura , vs. 17 or 18 in P. sucatio forms a significant and is a further distinguishing character ( Table 3). Notable differences are found in the proportional measurements of P. tenura compared to P. balitora , characters such as body depth, number of predorsal scales, circumferential scales, peduncle depth, preocciput length, interorbital width, head depth at occiput discriminate P. tenura from the other. The dorsal and lateral spots are distinct and discontinuous in P. tenura which in P. balitora is obscure and connected by a faint band. Psilorhynchus tenura is distinct from P. amplicephalus , a recently described species from northeastern India, in having lesser circumferential scales (15 vs 17–19), by its slender body (16.8–19.8% SL vs 23.2–25.8), shallow head region, its depth at pupil (43.3–46.6 vs 50.2–58.4) reduced head width (67.2–71.0% HL vs 74.1–84.4) and by the slender caudal peduncle, its depth (5.6–7.1% SL vs 8.1–9.8). To make out the resemblances shown by the new species with P. homaloptera and P. microphthalmus , the meristic data (Viswanath & Manojkumar, 1995) was used, since the type specimens could not be examined. The meristic data was very much enough to conclude that the new species is distinct from the other two species. Further P. tenura differs from P. microphthalmus by having variations in the series of spots along the lateral line, 8–9 vs. 10–12, and also the spots are said to be faint in latter, as given in the description which is dark in the new species.
Based on data from literature the three recently described species, P. arunachalensis Nabeshwar, Bagra & Das, 2007 , P. robustus Conway & Kottelat, 2007 and P. breviminor Conway & Mayden, 2008 , are also compared.
Psilorhynchus tenura , new species Psilorhynchus amplicephalus Psilorhynchus sucatio Psilorhynchus gracilis Psilorhynchus balitora
Holotype and paratypes Holotypes and paratypes UMMZ 205339 View Materials UMMZ 205343 View Materials UMMZ 207678 View Materials Characters N = 6 N = 9 N = 8 N = 5 N = 3
SL (mm)
Standard length 41.6–51.9 47.0 44.4–56.8 49.3 48.8–71.4 58.1 31.8–45.4 37.5 33.7–36.0 34.6 % SL
Predorsal length 46.8–48.0 47.3 48.6–53.9 51.4 43.8–47.7 45.8 46.9–49.2 48.2 46.6–50.3 48.1 Prepectoral fin 19.4–20.8 20.2 19.7–22.3 21.5 20.2–22.0 21.1 20.2–22.1 21.0 21.0–22.1 21.5 Body depth 16.8–19.8 18.4 23.2–25.8 24.4 19.2–22.5 21.2 21.5–23.7 22.8 21.8–22.0 21.9 Peduncle depth 5.6–7.1 6.4 8.1–9.8 8.9 6.4–7.4 6.8 8.8–9.5 9.1 8.7–9.0 8.9 Peduncle length 13.7–16.2 15.3 14.8–16.8 15.6 19.1–21.2 20.0 15.5–19.1 17.3 15.9–16.7 16.4 Pectoral fin length 24.3–28.5 26.3 17.5–19.6 18.7 18.9–23.0 20.6 23.8–25.4 24.5 29.4–29.8 29.5 Pelvic fin length 18.6–21.1 20.1 15.7–19.2 17.8 17.4–20.4 19.3 19.3–20.3 19.7 23.5–23.9 23.7 Anal fin length 12.6–14.2 13.5 15.1–17.4 16.8 12.5 –14.4 13.7 13.8–15.5 14.6 16.1–17.3 16.6 % HL
Head width 67.2–71.0 68.1 74.1–84.4 79.4 60.3–70.0 65.1 61.5–67.3 64.4 66.6–72.7 69.6 Snout to occiput 21.8–23.5 23.0 21.3–24.9 37.3 19.9–22.9 21.4 22.3–24.5 23.5 23.5–25.4 24.7 Orbit width 33.3–35.9 34.8 32.4–36.0 34.1 30.4–35.1 32.7 32.1- 37.3 35.4 30.4–34.7 32.5 Inter orbital width 31.6–34.8 33.1 34.5–41.7 37.9 44.9–50.6 48.3 31.2–33.5 32.1 34.0–37.6 35.8 Gape width 25.6–29.4 27.5 21.3–29.1 26.0 22.2–28.1 25.7 19.2–22.3 21.1 29.0–31.3 29.9 Mandible length 26.1–32.7 30.1 26.8–30.8 28.6 26.0–33.7 29.6 31.5–35.7 33.4 26.1–27.8 27.1 Head depth at pupil 43.3–46.6 45.4 50.2–58.4 54.9 38.5–42.7 40.1 43.1-50.2 45.4 49.1–51.1 50.3 Psilorhynchus tenura differs from P. arunachalensis in having lower number of lateral line scales (35–37 vs. 42–44), predorsal scales (11–12 vs. 13–18), lesser simple pectoral fin rays (5–6 vs. 8–9); from P. robustu s in the lateral line scale counts (35–37 vs. 32–34) and in having shallower body (16.8–19.8% SL vs. 18.8–22.7) and a slender caudal peduncle (5.6–7.1% SL vs. 7.5–8.4); from P. breviminor in lateral line scale count (35–37 vs. 31–32) by the shallow body (16.8–19.8% SL vs. 21.5–24.4), shorter predorsal length (46.8–48.0% SL vs. 50.0–55.0), slender and longer caudal peduncle, its depth (5.6–7.1% SL vs. 8.7–10.3) and its length (13.7–16.2% SL vs. 9.7–12.3) and reduced interorbital width (31.6–34.8% HL vs. 40.0–43.7) and by its lower number of rays in upper lobe of caudal fin (9 vs. 10). Principal Component analysis performed on the pooled measurements of specimens of four species examined demonstrated that the new species is distinct as evident in the biplot ( Fig. 2 View Fig ). The first three components explained about 87% variance, the first component (43.9% total variation) showed higher loadings in measures like body depth, peduncle depth and head depth at pupil.
Species of the Balitorid genera Bhavania , Travancoria, Balitoria and Homaloptera show superficial resemblance with the psilorhynchid species with the horizontally placed paired fins, inferior mouth and spots on the body; but they are distinct in many characters. Bhavania is distinguished in having a smaller gill opening, situated entirely above the pectoral base and Travancoria by the presence of deep rostral groove in front of mouth overhung by rostral fold with seven or more rostral barbels arranged in two lobes. Balitora is distinguished by blunt head, rostral flap divided into three lobes, presence of four short rostral barbels and a pair of maxillary barbels and lateral line with more than 60 scales.
after dissections and detailed study established that to be superficial. According to him the air bladder is not reduced to the extent as found in the other two genera Homoloptera or Bhavania .
The new species has adaptive features like much larger pectoral fins with more simple rays and a wider and higher body predorsally consistent to the nature of habitat it occurs. These fishes depress their head and force down onto their body to balance against strong water current. Pectoral fins with 5–6 unbranched rays, all with foreshortened fin ray segments (length <width a standard in Landberg & Marsh, 1976) serves as an adaptive character for fin substrate contact. Rainboth (1983) noted this shape as to be common among Asian hill stream fishes which attach themselves to hard substrate in high gradient streams (eg. Garra , Homaloptera , Gastromyzon ).
Ecology. – The fishes were collected from Korkanhalla, an east flowing stream, one of the main tributaries of river Thunga, which later confluence with Bhadra river as Thungabhadra. The stream is at an altitude of 652 m above mean sea level and the substrate types were cobble and gravel, the latter being in larger proportion. They are found attached to cobbles with their expanded pectoral fins and free swimming in shallow waters with medium flow. Other species encountered were Rasbora daniconius , Salmostoma boopis , Barilius canarensis , Garra mullya , Garra bicornuta , Puntius arulius , Osteochilichthys nashii , Puntius sahyadriensis (Cyprinidae) , Botia striata (Cobitidae) , Schistura semiarmatus , Balitora mysorensis ( Balitoridae ) Glyptothorax madraspatanam (Sisoridae) , Mystus keletius (Bagridae) and Parambassis ranga (Ambassidae) . Of this assemblage, Osteochilichthys nashii and Barilius canarensis were the most dominant species.
Homaloptera seemingly very much similar in general features with the Psilorhynchid species by the nature of paired fins, characters associated with the mouth, wider head region, spots on body, is distinguished by the presence of six barbels, four rostral and two maxillary and lips without papillae, strong jaws not covered by lips. Hora (1920), doubted the resemblance of this species with Homalopterid fishes but Discussion. – The distribution of the genus Psilorhynchus in India has been restricted to the north and northeastern states. Psilorhynchus balitora shows its distribution in all northeastern states except Nagaland, P. gracilis is present in lowlands of Assam and Mizoram, P. sucatio occurs in Meghalaya, Assam and Tripura and P. homaloptera in Meghalaya, Arunachal Pradesh and Nagaland states. In the Northeast region, Arunachal Pradesh is drained by Brahmaputra River and its tributaries whereas Assam is drained both by Brahmaputra and Barak and their tributaries. Eastern part of Northeast region comprising Nagaland, Mizoram and Manipur states is partly drained by Chindwin drainage ( Sen, 2000). Psilorhynchus balitora has been reported in all these regions except Nagaland. The Subansri, Dikrong, Pachin of western Arunachal Pradesh and the Siang river of eastern Arunachal Pradesh show the occurrence of P. balitora (Nath & Dey, 2000) . Though it is reported to be widely distributed in the Brahmaputra drainage, deterioration of water quality and habitat degradation have restricted populations to upper reaches of streams, one such case being the restricted population found in the Basistha, a hill stream of Assam (Das & Bordoloi, 1997). Psilorhynchus balitora is found in the Kosi and Jamuna tributaries of Ganges ( Menon, 1999) in the Northern Part of India. Johal et al. (1993) reported the occurrence of P. balitora in rivers of Rajasthan and also in Indus river systems. Psilorhynchus amplicephalus occurs in Balishwar river of Barak drainage in upper Assam ( Arunachalam et al., 2007) P. arunachalensis has been collected from Lohit, Siren, Kalphangi, Dikrong and Dirang rivers of Arunachal Pradesh (Nebeshwar et at., 2007).
David (1953) recorded Psilorhynchus sucatio damodari , a new geographical race of Psilorhychus sucatio , which is known to occur in the region of Eastern Himalayas and Northern Burma, from the Damodar river ( Fig.4 View Fig ). Rainboth (1983) presumed it to be a local race of P. sucatio which is a variant of individuals from Bangladesh. Being different from its eastern ally, the variety from Damodar was considered to be an isolate representative of this genus in the Peninsula. There have been no reports that show the presence of this genus in southern India except the inclusion of Psilorhynchus balitora by Jayaram (1995), in the fish distribution list of his report on bioresources of river Krishna. According to him the species was found to occur in upstream of Krishna above Dhom dam near Ondhisi village in Satara district of Maharashtra, in which he also mentions the earlier report of the species by David (1964) from the same locality. However, in either case, no details are available of how many specimens were collected and where they were deposited. Also, no specimens of Psilorhynchus were collected during the extensive ichthyological survey made in the headwaters of Krishna above Dhom dam by the first author and team in 1997. Since no literature has confirmed the presence of this species substantially and also as this region has not been included in the range of distribution of this genus, we consider this report as an extension of range to streams of peninsular India. Further we suggest that a thorough survey is required to confirm the occurrence of this genus in other headwater streams and lowland reaches of rivers in peninsular India.
On the zoogeographical significance, a number of fishes from Indo-Chinese fauna are found within the characterised fauna of the peninsula. Hora’s (1942, 1944) Satpura hypothesis was mainly based on the distribution of forms like Osteochilus, Neolissochilius , Schismatorhynchus , Rohtee , Batasio and homalopterids ( Balitora ) that were recognized as pre-tilt forms. Recently the genus Osteochilus has been shown to be distinct from species occurring in peninsular Indian region ( Osteochilichthys nashii , O. thomassi and O. longidorsalis ). Kottelat (1989) and Karnasuta (1993) clearly stated that Osteochilichthys belongs to the subfamily Barbinae while Osteochilus is a member of Labeoninae . Another example of Hora’s hypothesis is the distribution of Schismatorhynchus ( Nukta) from peninsular India showing the relationship of Schismatorhynchus from Borneo. However, a revision of the genus ( Reid, 1982; Siebert & Tjakrawidjaja, 1998) showed that Schismatorhynchus from Borneo is distinct from Hora’s subgenus Schismatorhynchus ( Nukta) hence this genus from peninsular India is Nukta. Endemic genera like Horalabiosa , Bhavania , Travancoria and Hypselobarbus need further reasoning to substantiate the hypothesis. The identity of Homalopterid species in South India remains doubtful (Pethiyagoda & Kottelat, 1994). These fishes are said to have evolved during later tertiary lines ( Mani, 1974) and the affinity between Western Ghats and northeastern Himalayas merely represents the relic fauna ( Ripley, 1949) and most of the peninsular fauna had been considerably influenced by the extra peninsular forms (Mani, 1941).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |