Neanthes visicete, Georgieva & Wiklund & Ramos & Neal & Glasby & Gunton, 2023
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.75.2023.1800 |
|
DOI |
https://doi.org/10.5281/zenodo.11003019 |
|
persistent identifier |
https://treatment.plazi.org/id/E679B631-FFA0-FF8F-84F7-FF29FA6AFE6F |
|
treatment provided by |
Felipe |
|
scientific name |
Neanthes visicete |
| status |
sp. nov. |
Neanthes visicete View in CoL sp. nov.
urn:lsid:zoobank.org:act:18CD5A8D-DFB6-4E46-9694-FA3EC5D5E51E
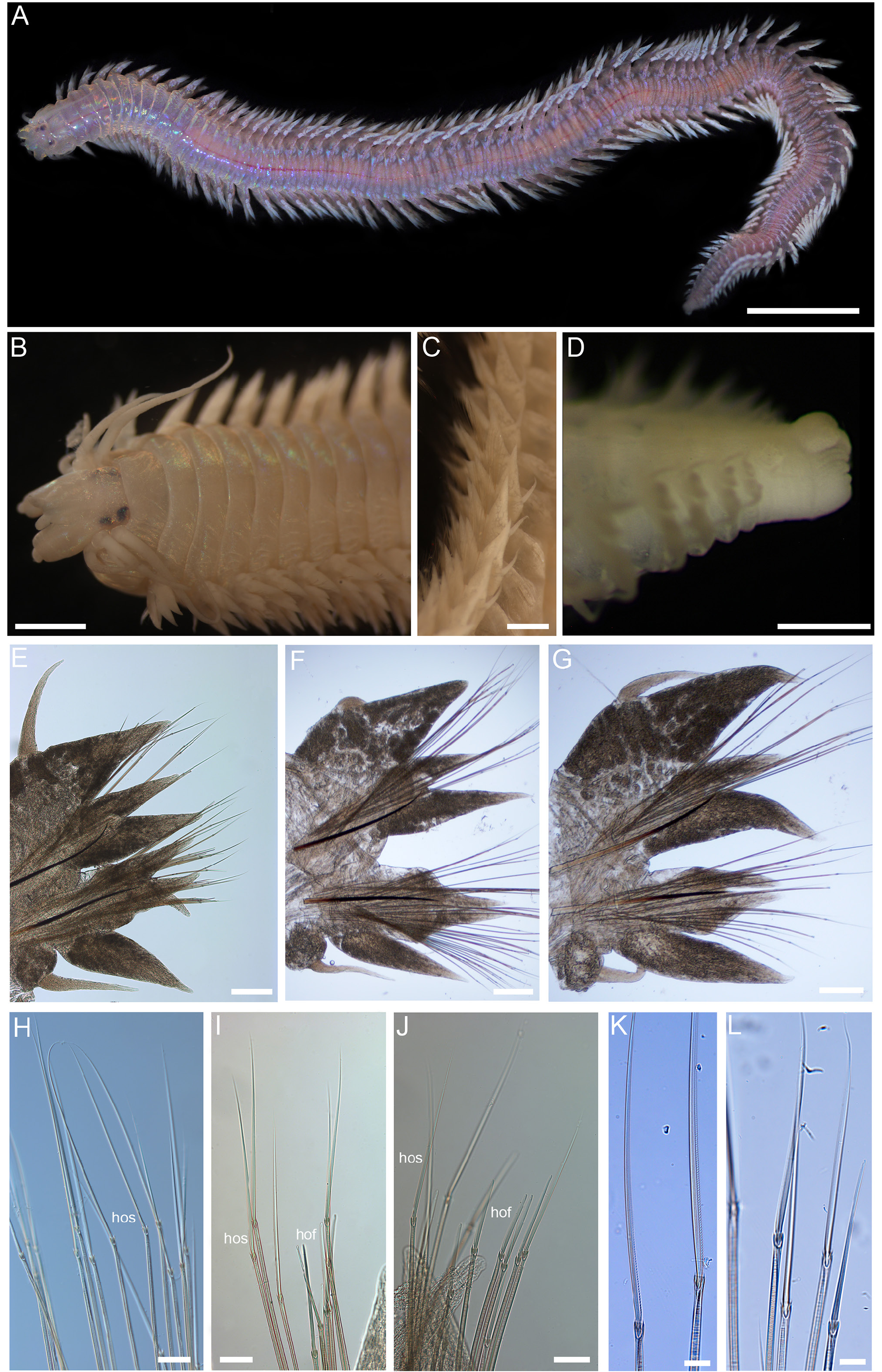
Fig. 15 View Figure 15
Neanthes sp. 1 . Gunton et al., 2021: 70–71, fig. 15C, D
Holotype: AMW.53704, IN2017_ V03 _100; 9 June 2017; off Byron Bay , NSW, Australia, beam trawl, start: 28.05°S 154.08°E, 999 m, end: 28.10°S 154.08°E, 1013 m GoogleMaps . Paratype: AMW.52209, samelocalityas holotype. GoogleMaps DNA vouchers: AM W.53704 ( COI, 16 S, 18 S), GoogleMaps AMW.52209 (16 S). GoogleMaps
Description. Holotype complete, pinkish-purplewhen alive, with whitish notopodial ligules and noticeable iridescence ( Fig. 15A View Figure 15 ); 85 chaetigers, upto 50 mm long and a maximum of 2.6 mm wide (without parapodia), tapering towards posterior.
Prostomium ( Fig. 15B View Figure 15 ) trapezoidal, longer than wide, with a dorsaldepression thatextends fromanterior tip tojust below eyes. One pair of short antennae approximately onequarter lengthof prostomium and onepair of elongated palps with cylindrical palpophore and oval-shaped palpostyle, extending just beyond antennae. Two pairs of eyes, anterior pair roughly oval-shaped, posterior pair slightly larger, kidney-shaped, and slightly closer together.
Tentacular belt (first adult annulus) slightly longer than first chaetiger, with four pairs of tentacular cirri each with distinct cirrophores; posterodorsal pair longest, extending back to fifth chaetiger. Pharynx with smooth brown jaws and 7 or 8 teeth on cutting edge, paragnaths all conical. Paragnath arrangement as follows: Areas I—13 paragnaths; II—rectangular clusterof ≥30 paragnaths; III—widely spaced rectangular cluster of c. 45 paragnaths; IV—dense triangular cluster of c. 50 paragnaths; V—none; VI—small circular cluster of 6–8 paragnaths; VII–VIII—strip of c. 50 paragnaths arranged in 2 or 3 rows.
Chaetigers 1–2 sub-biramous (notoaciculae absent), followed by biramous chaetigers. Sub-biramous chaetigers withdorsalcirri slightly shorter thandorsal notopodial ligule; dorsal cirri inserted at base of ligule. Dorsal notopodial ligule of similar shape and length as ventral notopodial ligule; conical and slightlyshorter than neuroacicular ligule. Ventral cirri slightly shorter than ventral neuropodial ligule (two-thirds of length).
Parapodia of biramous chaetigers ( Fig. 15C, E–G View Figure 15 ) with notopodia larger than neuropodia, notopodial dorsal cirri inserted at base of, and slightly shorter than (around two-thirds length) or same length as dorsal notopodia ligules. Notopodium consisting of three similar-sized ligules/lobes: dorsal notopodial ligule conical and prominent, largest structure of parapodia; prechaetal notopodial lobe slightly smallerthandorsalnotopodialliguleanteriorly, ⅔ itslength in mid-body and ½ its length posteriorly; ventral notopodial ligule conical, slightly smaller than dorsal notopodial ligule throughout. Neuropodia with three distinct lobes/ligules: prechaetal neuropodial lobe conical, approximately ⅔ lengthof postchaetallobe anteriorly and posteriorly, pre- and postchaetal lobes approximately equal in size in mid-body; ventral ligule conical, extending just short of pre- and postchaetal lobes in anterior and mid-body, equal to those lobes in posterior body.
Notochaetae ( Fig. 15H View Figure 15 ) allhomogomph spinigersarising from supra-acicular fascicle. Neurochaetae ( Fig. 15I–L View Figure 15 ) arranged in sub- and supa-acicular fascicles, both with homogomph spinigers; homogomph (and sesquigomph) falcigers present in both fascicles anteriorly, falcigers only present inventral fascicle inmid-body and byposteriorbody absent altogether. Blades of spinigers and falcigers finely serrated ( Fig. 15K–L View Figure 15 ); spinigerblade lengthdecreasing from dorsal to ventral side; falciger blades elongate, unidentate, and very finely serrated along entire length. Dark noto- and neuroaciculae present in each ramus of the biramous parapodia ( Fig. 15E–G View Figure 15 ); notoaciculae slightlycurvedupward distally; neuroaciculae more or less straight.
In anterior parapodia (represented by chaetiger 13 and 14): notochaetae comprising 12–14 homogomph spinigers per fascicle; supra-acicular neurochaetae comprising 8–10 homogomph spinigers and 3–4 homogomph/sesquigomph falcigers; sub-acicular neurochaetae comprising 7–13 homogomph spinigers and 6–8 homogomph/sesquigomph falcigers. In mid-body parapodia (represented by chaetiger 28): notochaetae comprising 21 homogomph spinigers per fascicle; supra-acicular neurochaetae comprising 12 homogomph spinigers (homogomph/sesquigomph falcigers have disappeared); sub-acicular neurochaetae comprising 16 homogomph spinigers and 3 homogomph/sesquigomph falcigers. In posterior parapodia (represented by chaetiger 67): notochaetae comprising 14 homogomph spinigers per fascicle; supra-acicular neurochaetae comprising 7 homogomph spinigers (homogomph/sesquigomph falcigers not present; sub-acicular neurochaetae comprising 10 homogomph spinigers (homogomph/sesquigomph falcigers not present).
Pygidium ( Fig. 15D View Figure 15 ) with distinct ventral lobe, pygidial cirri probably absent (no obvious cirri scars on the pygidial rim or ventral lobe) or lost.
Distribution. IN2017_V03, Station 100. Pilot whale carcass, off Byron Bay, New South Wales, Australia in 999–1013 m.
Etymology. Namedderived from the Latin root “visitar” for a visitor, and “cete”, a whale, referring to the new species’ occurrence on a whale fall. Noun in apposition.
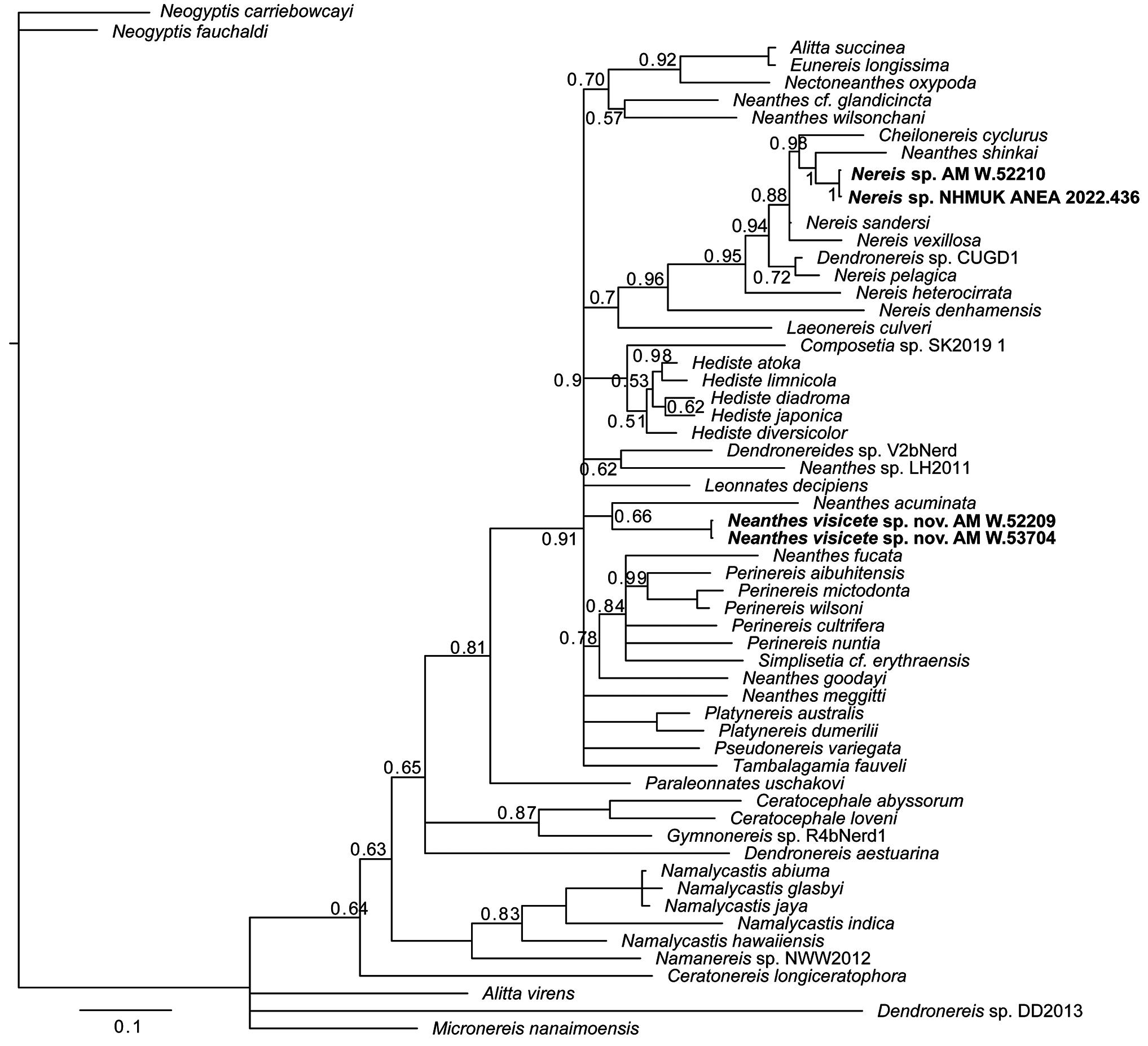
Remarks. Our Nereididae molecular phylogeny ( Fig. 16 View Figure 16 ) resolves Neanthes visicete sp. nov. in a cladewith Neanthes acuminata Ehlers, 1868 , however, with poor support. While a Nereididae phylogenetic analysis by Villalobos-Guerrero et al. (2022) recovered Alitta and Nectoneanthes in a clade with Neanthes acuminata , Alitta , and Nectoneanthes occurred in a separate clade in our phylogenetic analysis, perhaps due to the different genetic markers included. No other known Nereididae species for which genetic data are available appear to be genetically closely related to N. visicete sp. nov., with genetic distances being a minimum of 17.7% between N. visicete sp. nov. and other Nereis and Neanthes species for which genetic data is available (Table S16). Neanthes acuminata is recognized as a species complex, of which the species Neanthes arenaceodentata ( Moore, 1903) and Neanthes cricognatha ( Ehlers, 1904) are also a part ( Reish et al., 2014). Of these, N. cricognatha is the only species to have been reported from off Australia, including a recent record at 1194–1257 m depth from the IN2017_V03 expedition ( Gunton et al., 2021). Neanthes visicete sp. nov. differs from the IN2017_V03 N. cricognatha specimens visibly due to its whitish notopodia in living specimens, and a different paragnath arrangement of Areas V–VIII (broad continuous band in the latter, areas discrete in the new species).
A few other species of Neanthes have been recorded from deep waters off Australia. Neanthes cf. bassi Wilson, 1984 was also recorded from the IN2017_V03 expedition and recent voyages to the Great Australian Bight at depths of 200–4800 m ( Gunton et al., 2021). Neanthes bassi , Neanthes tasmani Bakken, 2002 and N. kerguelensis are morphologically very similar, however in comparison to N. visicete sp. nov., none of these species have the whitish grainy notopodia that appear characteristic of N. visicete sp. nov. Additional differences are that N. bassi has smooth bars in pharyngeal Area IV ( Wilson 1984) while both N. kerguelensis and N. tasmani have fewerparagnaths in Areas VI–VIII ( Bakken, 2002); N. kerguelensis additionally has longer tentacular cirri than N. visicete sp. nov. Finally, all three species have heterogomph falcigers, whereas they are absent from the new species. Neanthes heteroculata ( Hartmann-Schröder, 1981) was also recorded from the IN2017_V03 voyage at 3980–4280 m depth ( Gunton et al., 2021), but these specimens have very large eyes and are thus again clearly distinguishable from N. visicete sp. nov.
Neanthes visicete sp. nov. and Neanthes adriangloveri sp. nov. are only the second and third formally described Neanthes to be found associated with a whale fall. The first, N. shinkai , was described from abyssal depths of the southwest Atlantic. This species is quite different from the two new Neanthes species described here in lacking prechaetal notopodial lobes, postchaetal neuropodial lobes and eyes; in these features and in our molecular phylogeny N. shinkai more closely resembles our Nereis sp. (see following account). Shimabukuro et al. (2017) analysed the carbon and nitrogen isotopes of N. shinkai and concluded that it was an omnivore that was feeding mainly on the organic matter from the whale.
Comparative morphology. Although Neanthes is one of the most species-rich genera of Nereididae with about 80 valid species, only 25 species share with N. visicete sp. nov. some important parapodial features including dorsal notopodial ligule that is similar sized along the body (as opposed to enlarged posteriorly), presence of prechaetal notopodial lobes and presence of a postchaetal neuropodial lobe ( Villalobos-Guerrero & Idris, 2021, table 2). This group of 25 can be narrowed down to nine by including an unusual feature of the new species, the presence of homogomph spinigers in the sub-acicular fascicle of the neuropodia: N. acuminata , N. arenaceodentata , N. articulata , Neanthes chingrighattensis ( Fauvel, 1932) , N. cricognatha , N. kerguelensis , N. picteti ( Malaquin & Dehorne, 1907) , N. pleijeli de León-González & Salazar-Vallejo, 2003 and N. suluensis . Considering the paragnath numbers of these nine species, the new species is—as also found using molecular data—closest to Neanthes acuminata , N. arenaceodentata and N. cricognatha , butdiffers inhaving a greater numberof paragnaths inArea III (c. 45 vs 23–28 in acuminata ; 20–34 in N. cricognatha ), and fewer inthis areathan arenaceodentata (82) and having paragnaths absent in Area V, vs present and merging with a broad band of paragnaths in Areas VII–VIII in N. acuminata , N. arenaceodentata and N. cricognatha . Neanthes visicete sp. nov. has a high number of paragnaths in Area I (13) which distinguishes it from N. articulata (1), N. chingrighattensis (2–6; chingrighattensis also has the unusual presence of a neuropodial superior lobe which is absent in the new species), N. kerguelensis (0–4), N. picteti (2) and N. pleijeli (2). The new species can be distinguished from the poorly known N. suluensis by having 2 or 3 rows of paragnaths in Areas VII–VIII (only 1 in suluensis ). Finally, the new speciescan be distinguishedfrom all known Neanthes species by lacking heterogomph falcigers, by its distinct ventral pygidial lobe (although pygidial features are poorly known in Nereididae ), and its distinctive living colouration (pinkish-purple with whitish dorsal notopodial ligules and noticeable iridescence).
The presence of a large prechaetal notopodial lobes throughout thebody in thenewspecies (and the N. acuminata species complex), such that the notopodia appear to have three similar-sized lobes/ligules, also occurs in Alitta , Nectoneanthes and Leonnates ( Bakken, 2006; Bakken et al., 2022). One might therefore question the placement of the new speciesin Neanthes consideringits lackof heterogomph falcigers; however, the new species is here treated as a Neanthes because itlacks thepresence of anexpanded dorsal notopodial ligule of Alitta , it lacks the ovoid lobe above the dorsal cirrus of Nectoneanthes , and the oral ring papillae of Leonnates .
Although closest to Neanthes in overall morphology (because the concept of Neanthes is currently so broad), the species does not fit the current definition of Neanthes (Bakken et al., 2022; Villalobos-Guerrero et al., 2022), because of its lack of neuropodial heterogomph falcigers. It has homogomph and sesquigomph falcigers, and they are restricted to anterior and mid-body chaetigers. This emendation is best made in a future revision of the genus.
| AMW |
Australian Museum |
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Neanthes visicete
| Georgieva, Magdalena N., Wiklund, Helena, Ramos, Dino A., Neal, Lenka, Glasby, Christopher J. & Gunton, Laetitia M. 2023 |
Neanthes sp. 1
| Gunton, L. M. & E. K. Kupriyanova & T. Alvestad & L. Avery & J. A. Blake & O. Biriukova & M. Boggemann 2021: 70 |