Milnesium pelufforum, Rocha & González-Reyes & Ostertag & Lisi, 2022
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.822.1807 |
|
publication LSID |
lsid:zoobank.org:pub:522FD009-B4C9-4A80-871E-883E2EBE09C8 |
|
DOI |
https://doi.org/10.5281/zenodo.6620642 |
|
persistent identifier |
https://treatment.plazi.org/id/0DA71F31-0FA3-4F27-91EC-CC028C5AC808 |
|
taxon LSID |
lsid:zoobank.org:act:0DA71F31-0FA3-4F27-91EC-CC028C5AC808 |
|
treatment provided by |
Felipe |
|
scientific name |
Milnesium pelufforum |
| status |
sp. nov. |
Milnesium pelufforum View in CoL sp. nov.
urn:lsid:zoobank.org:act:0DA71F31-0FA3-4F27-91EC-CC028C5AC808
Figs 1–12 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig , Tables 1–5 View Table 1 View Table 2 View Table 3 View Table 4 View Table 5
Diagnosis
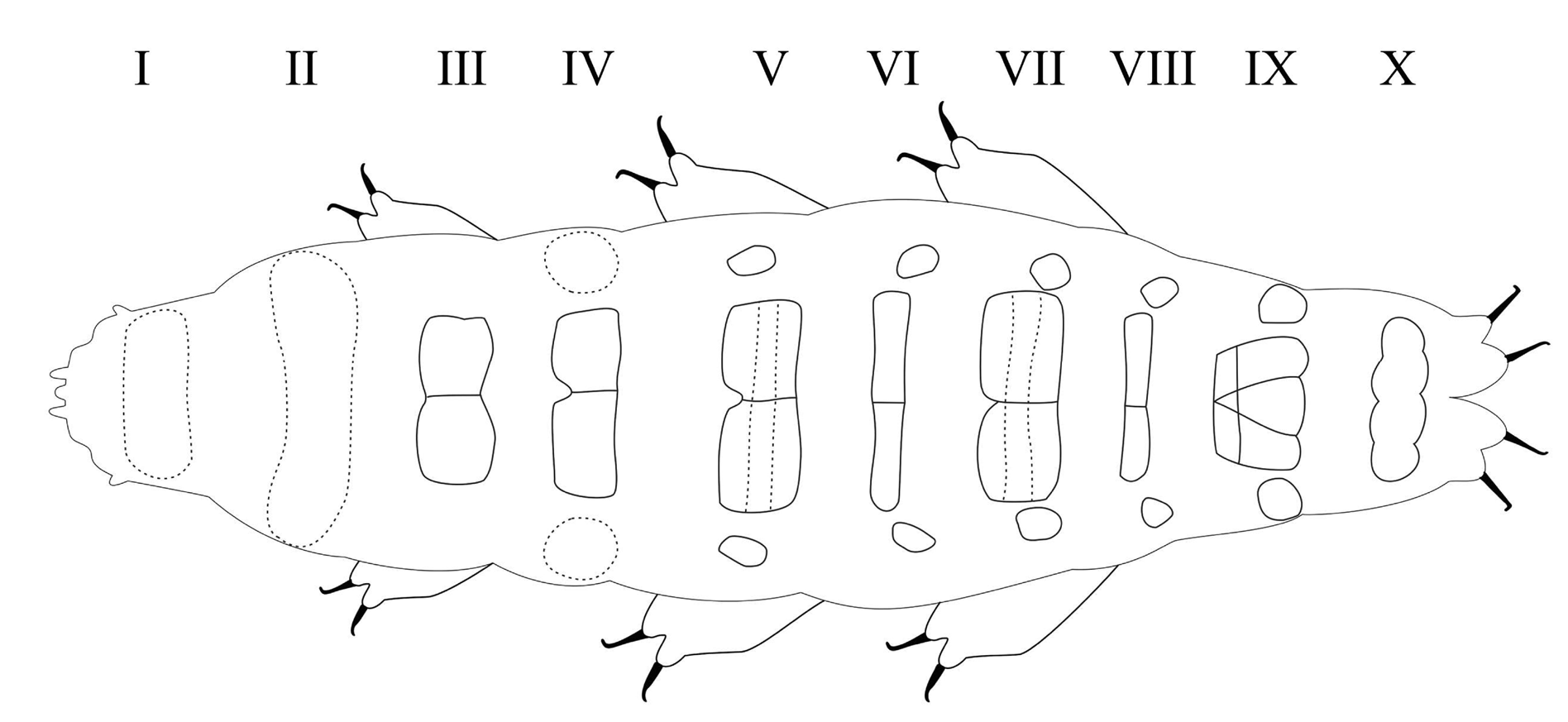
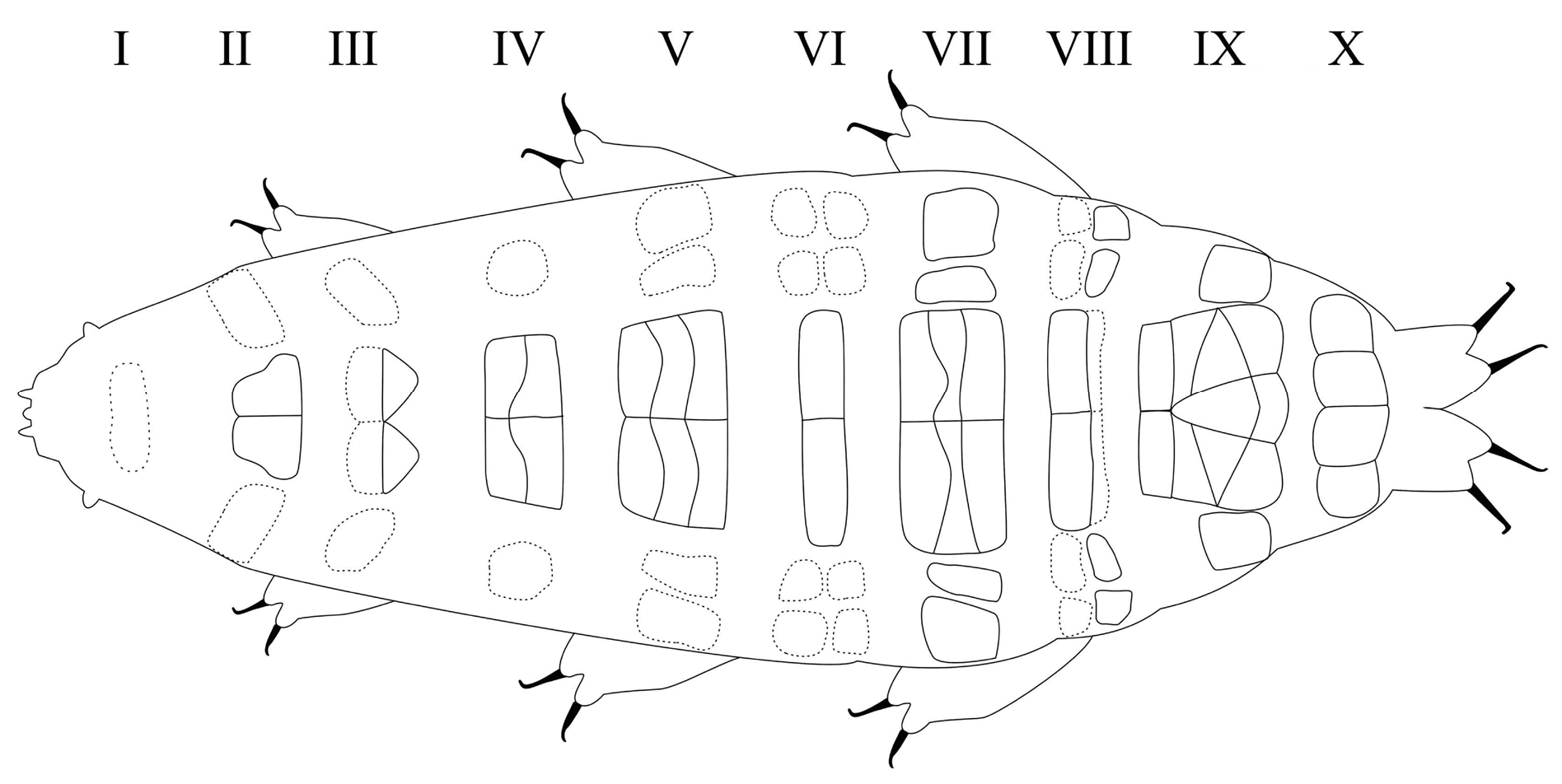
Ten transverse bands, better defined in senior specimens, of sculptured cuticle consisting of dimples forming a reticular pattern. Dimples, larger in young than in senior specimens, often showing some internal structure; ten rows of pseudoplates also present, better outlined in senior specimens, formula CP: I:1; II:4; III:6; IV:6; V:10; VI:10; VII:10; VIII:12; IX:12; X:4. Pseudoplate rows coincide with sculptured bands but sculpturing is not limited only on pseudoplates.
Six peribuccal lamellae, six peribuccal papillae equal in size, two lateral cephalic papillae. Buccal tube nearly cylindrical, wider in senior specimens; claw configuration [2-2]-[2-2] in young, [2-3]-[3-2] in senior specimens (but with very small basal spurs, especially on legs IV).
Etymology
The new species is dedicated to Maria Cristina Moly de Peluffo and Julio Ricardo Peluffo , the first researchers of tardigrades from the National University of La Pampa, Argentina.
Material examined
Holotype ARGENTINA • ♀; Salta Province, Salta City; 24°47′18″ S, 65°24′38″ W, 1150 m a.s.l.; 2 May 2014; Rocha-Doma leg.; moss and lichens from trees; MCNS Tar.000021(3) . GoogleMaps
Paratypes ARGENTINA • 2 ♀♀; same collection data as for holotype; MCNS Tar.000021(2) , Tar.000021(4) GoogleMaps • 4 ♀♀; same collection data as for holotype; UNICT 5898(1) to 5898(4) GoogleMaps • 17 ♀♀; same collection data as for holotype; UNLPam 389(1) to 391(3) , 454(1) to 454(5) , 465(1) to 465(4) , 456(4) to 456(5) , 503(1) to 503(3) GoogleMaps .
Morphological description
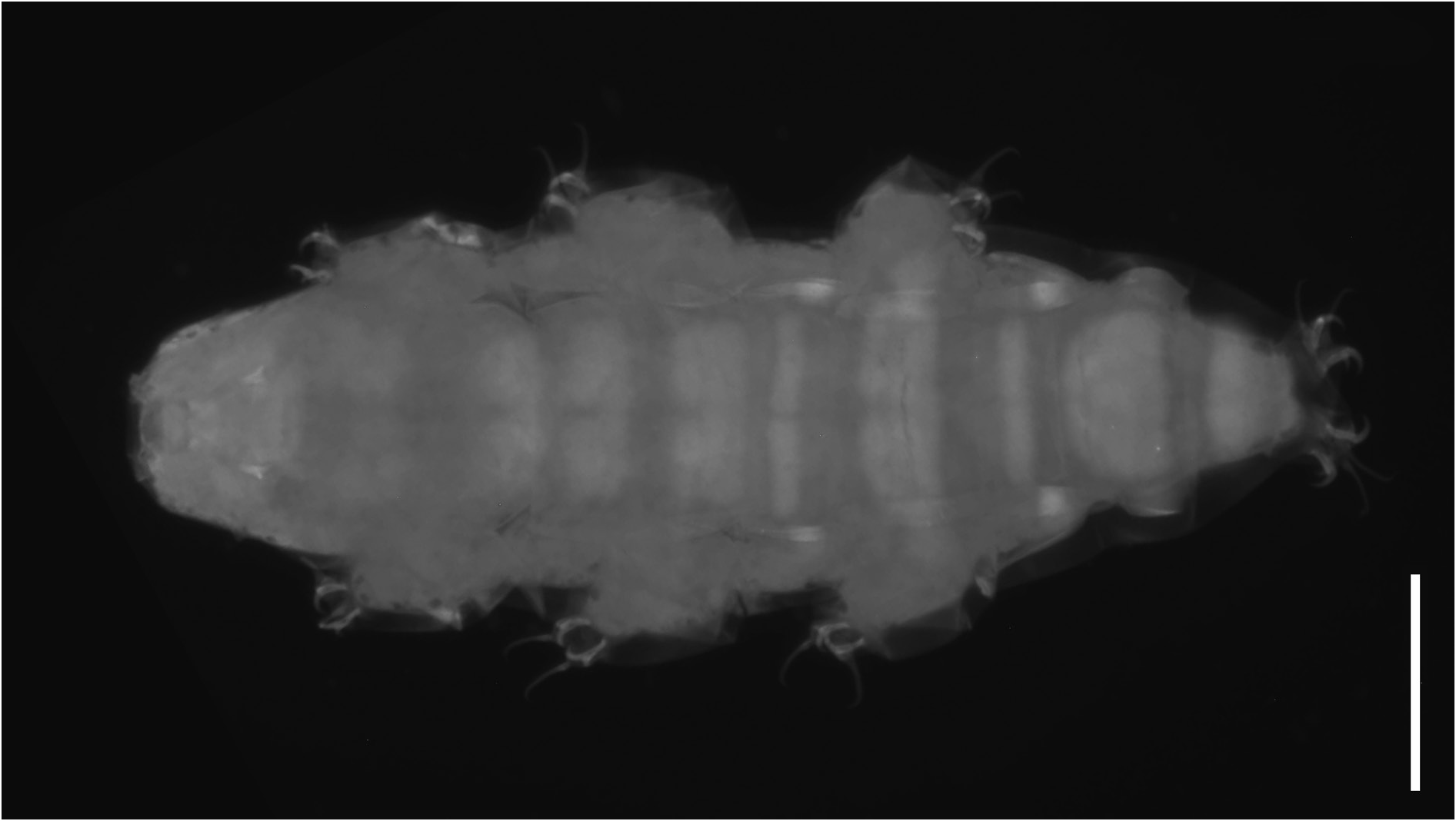
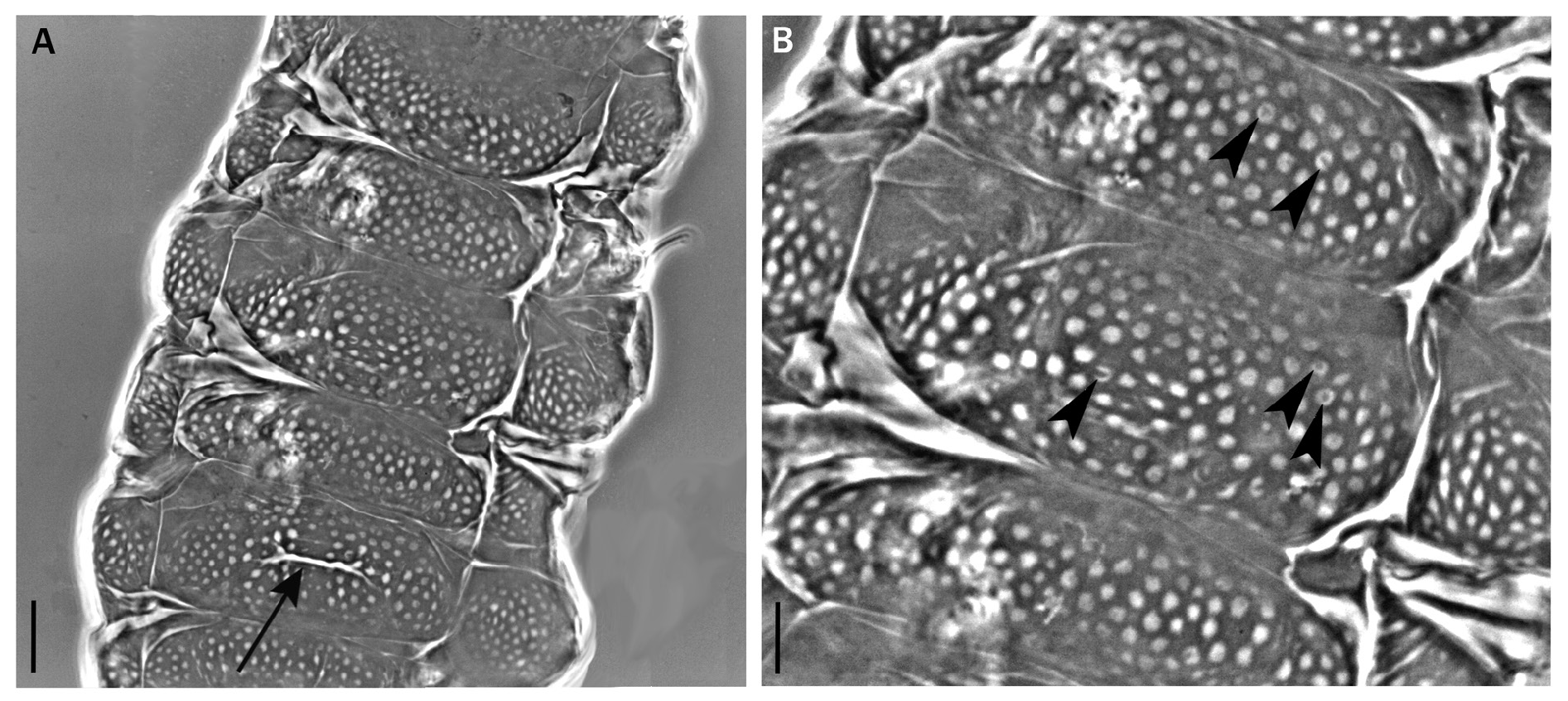
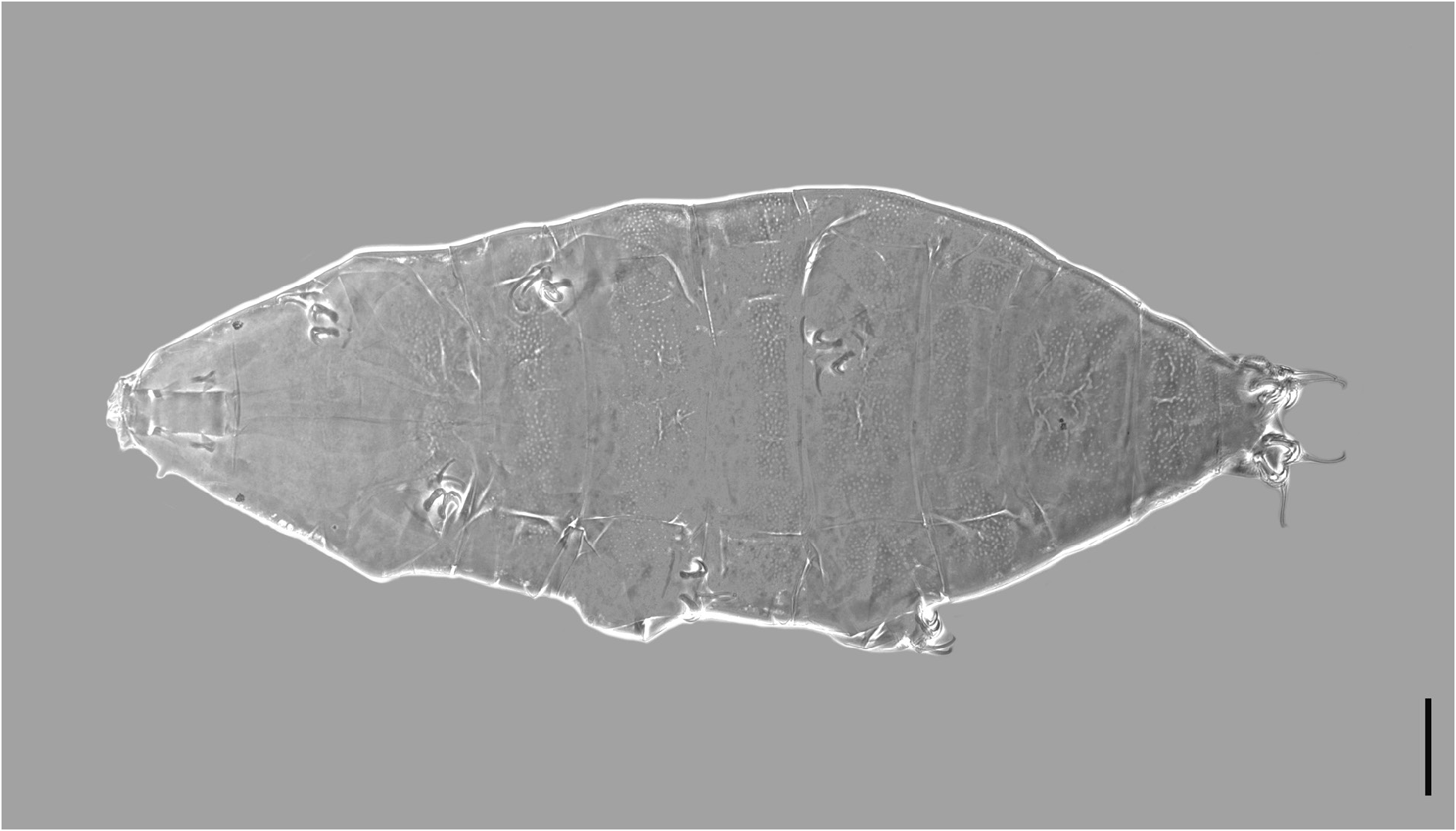
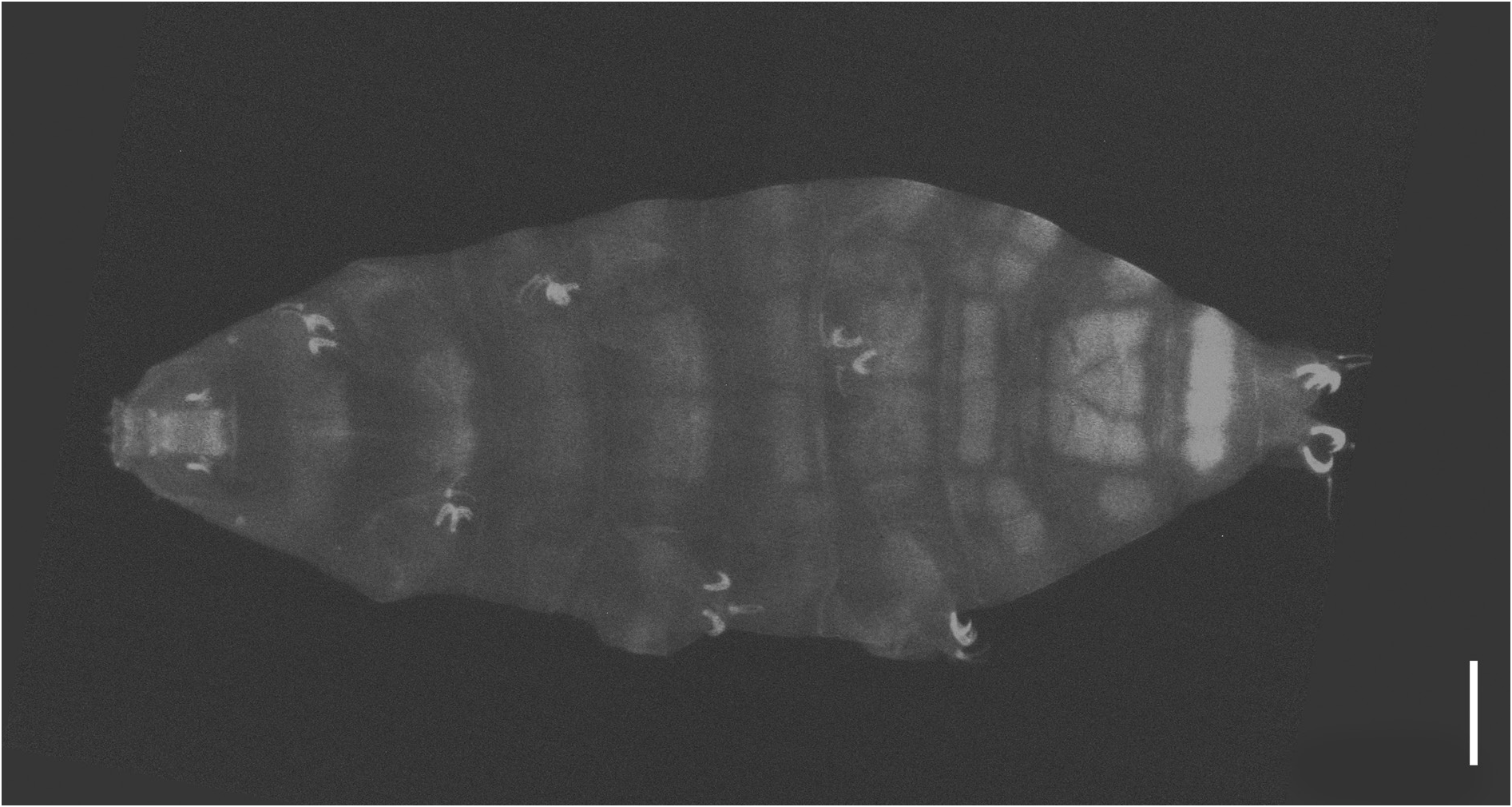
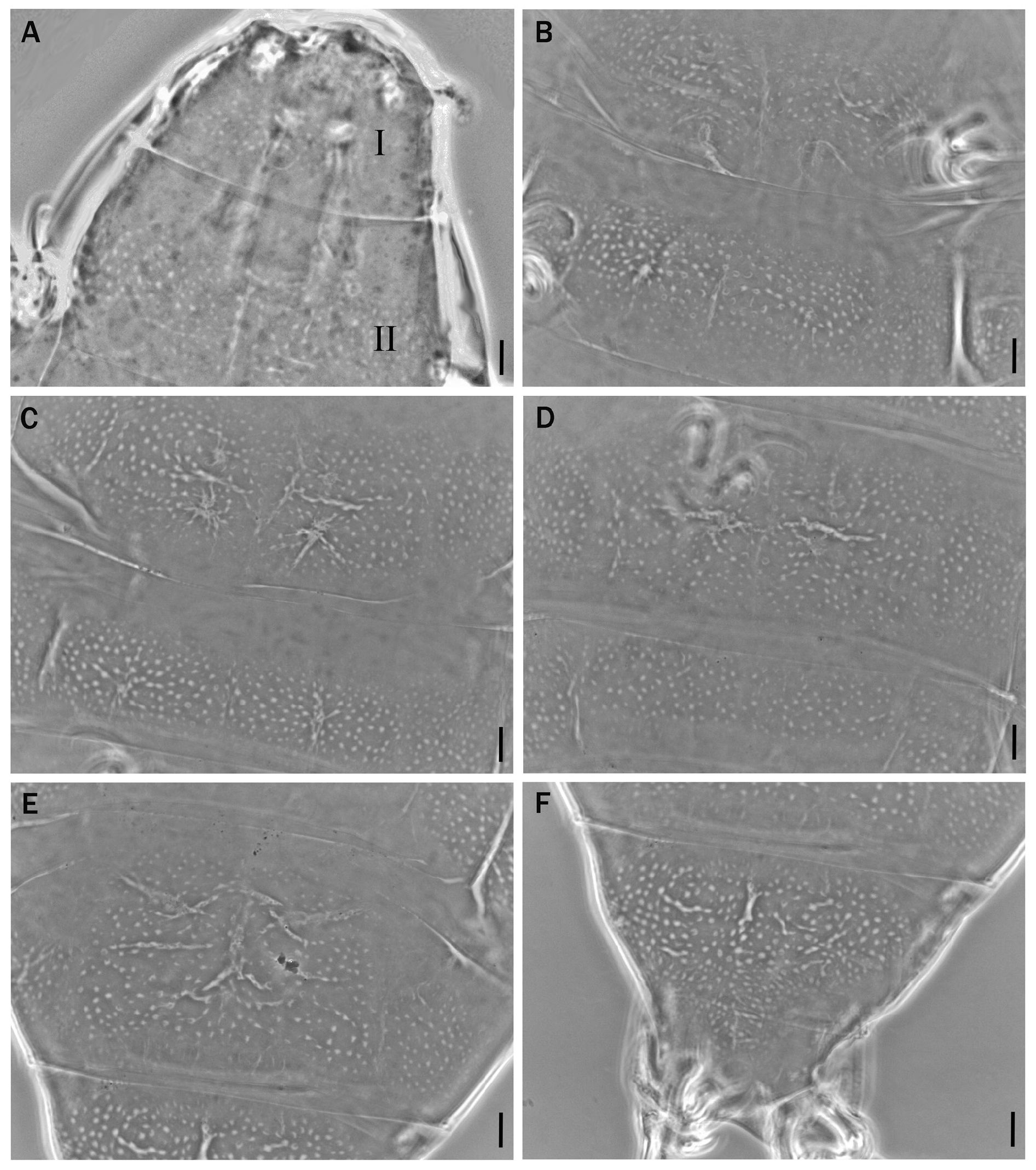
Body length from 216 µm to 620 µm, reddish colour before mounting, eyes present (habitus in Figs 1 View Fig , 3 View Fig , 8 View Fig ). Dorsal and dorsolateral cuticle with variously shaped dimples (depressions) ( Figs 1–3 View Fig View Fig View Fig , 6 View Fig , 11 View Fig ) forming a reticular pattern, arranged in ten transverse bands (as a tendency in young, as a rule in senior specimens), one band on each of the ten subsegments of the body ( Figs 1 View Fig , 3 View Fig , 11 View Fig ) including the very first, cephalic one; dimples often showing internal structures of variable appearance ( Figs 2 View Fig , 6 View Fig , 11 View Fig ), but further investigations are required to ascertain whether apparent presence or absence, and shape, of such internal structures is just due to matter of focus under the microscope and orientation of the structures in the preparation; dimples vary in size but not in shape or details of internal structure, between young and senior specimens. Pseudoplates, better outlined in senior specimens, present ( Figs 4–5 View Fig View Fig , 9–10 View Fig View Fig ), also arranged in ten transverse bands (again, including the very first, cephalic subsegment); pseudoplate rows correspond with the sculptured bands but the sculpturing is not limited only on pseudoplates; pseudoplate formula is CP: I:1; II:4; III:6; IV:6; V:10; VI:10; VII:10; VIII:12; IX:12; X:4 (based on senior specimens). Row I is situated at the level of the buccal tube and has only one medial pseudoplate, difficult to see, more or less rectangular, laying transversally, with rounded angles. Row II, situated just anteriorly to legs I, has four pseudoplates: two medial, about trapezoidal, touching in the central line along their longer side, and two separate lateral, about rectangular, laying obliquely and difficult to see. Row III, situated in line with legs I, has six pseudoplates: four central arranged in two pairs, all four connected, with the two anterior, difficult to see, about rectangular, and the two caudal, triangular, pointing backwards; two lateral pseudoplates, about ellyptical, laying obliquely and difficult to see. Row IV, situated between legs I and II, has six pseudoplates: the four medial arranged in two pairs, transversally elongated, connected, forming a unique about rectangular structure; each of these four pseudoplates vaguely rectangular but with the transverse line dividing the anterior and posterior couple not straight; two rounded lateral pseudoplates, difficult to see. Row V, situated in line with legs II, has ten pseudoplates: the six medial arranged in three pairs, transversally elongated, connected, forming a unique about rectangular/trapezoidal structure; each of these six pseudoplates vaguely rectangular but with the two transverse lines dividing the adjacent anterior/posterior pairs of pseudoplates not straight; lateral to that central complex, on each side, two separate pseudoplates, difficult to see, with the midlateral less developed and vaguely in the shape of a curved trapezium, while the most lateral is more developed and vaguely quadrangular. Row VI, situated between legs II and III, has ten pseudoplates: two medial rectangular, sided laterally and very elongated transversally; lateral to them, on each side, four separate pseudoplates difficult to see, arranged in a quadrangle. Row VII, situated in line with legs III, has ten pseudoplates: the six medial arranged in three pairs, transversally elongated, connected, forming a unique about rectangular structure; each of these six pseudoplates vaguely rectangular but with the two transverse lines dividing the adjacent anterior/posterior pairs of pseudoplates not straight; lateral to that central complex, on each side, two separate pseudoplates, with the mid-lateral less developed and vaguely in the shape of a curved trapezium, while the most lateral is more developed and vaguely quadrangular. Row VIII, situated just posterior to legs III, has twelve pseudoplates with the four medial arranged in two pairs, transversally elongated, connected, forming a unique about rectangular structure; each of these four pseudoplates vaguely rectangular but with the two anterior well visible and not thin, while the two posterior difficult to see and very thin; lateral to that central complex, on each side, four separate pseudoplates arranged in a quadrangle: the two mid-lateral elongated transversally, the two very lateral vaguely quadrangular; besides, the two anterior pseudoplates of the tetrad difficult to see, while the two posterior better visible. Row IX, situated between legs III and IV, has twelve pseudoplates with a central aggregation of 10 pseudoplates forming a complex pattern (see Fig. 10 View Fig ), and two lateral single pseudoplates about quadrangular, aligned mid-posteriorly. Row X, situated just anterior to legs IV, has four about quadrangular pseudoplates, sided and aligned in a single row transversally.
Cuticular grooves present dorsally, but this and other details of the cuticular ornamentation vary depending on life stage (see below).
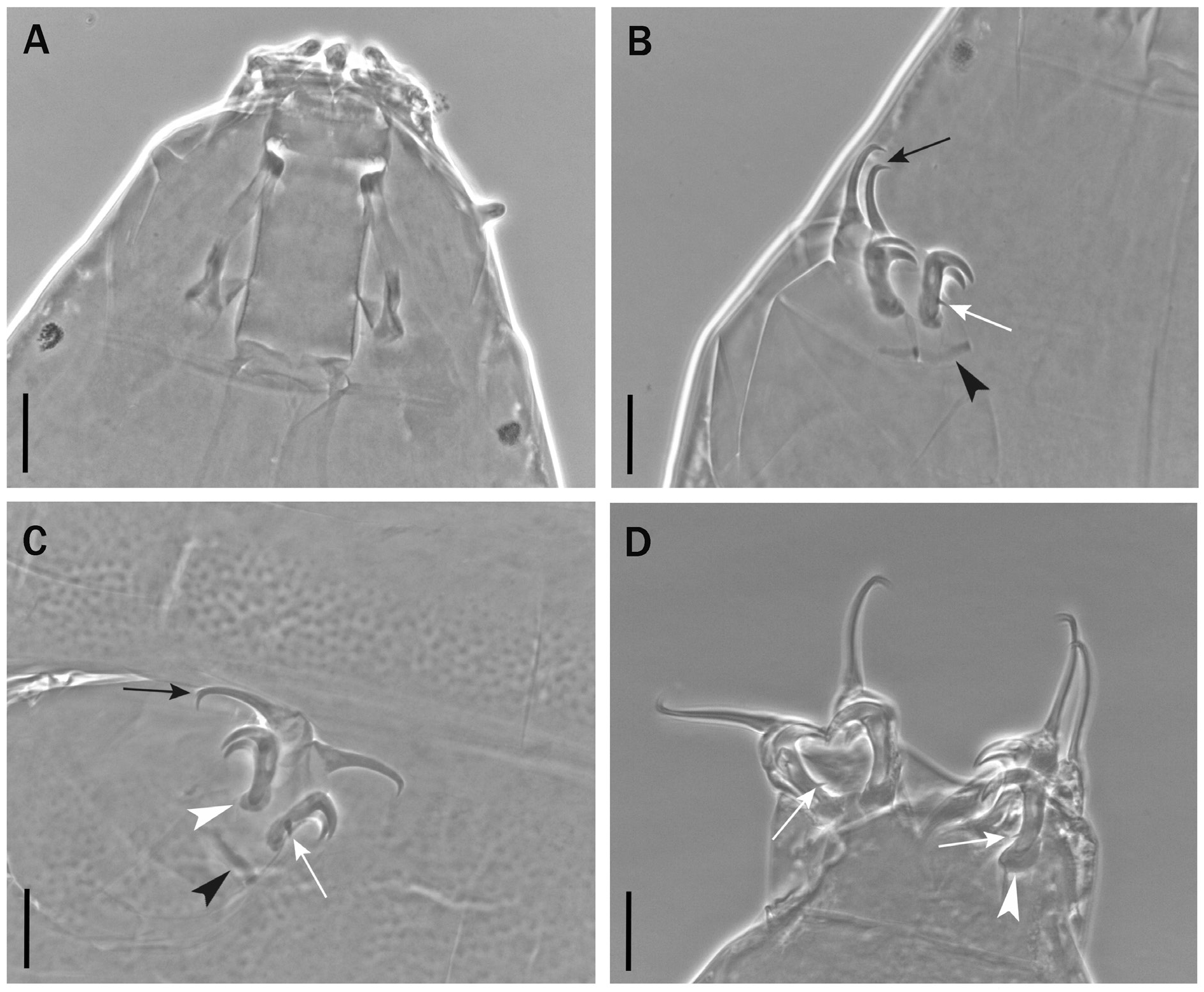
Six peribuccal lamellae and six peribuccal papillae plus two lateral papillae present. Buccal tube ( Figs 7A View Fig , 12A View Fig ) nearly cylindrical (posterior/anterior width ratio 82–100%), more slender in young specimens; pt of the stylet support insertion point on the buccal tube [65.0–73.8].
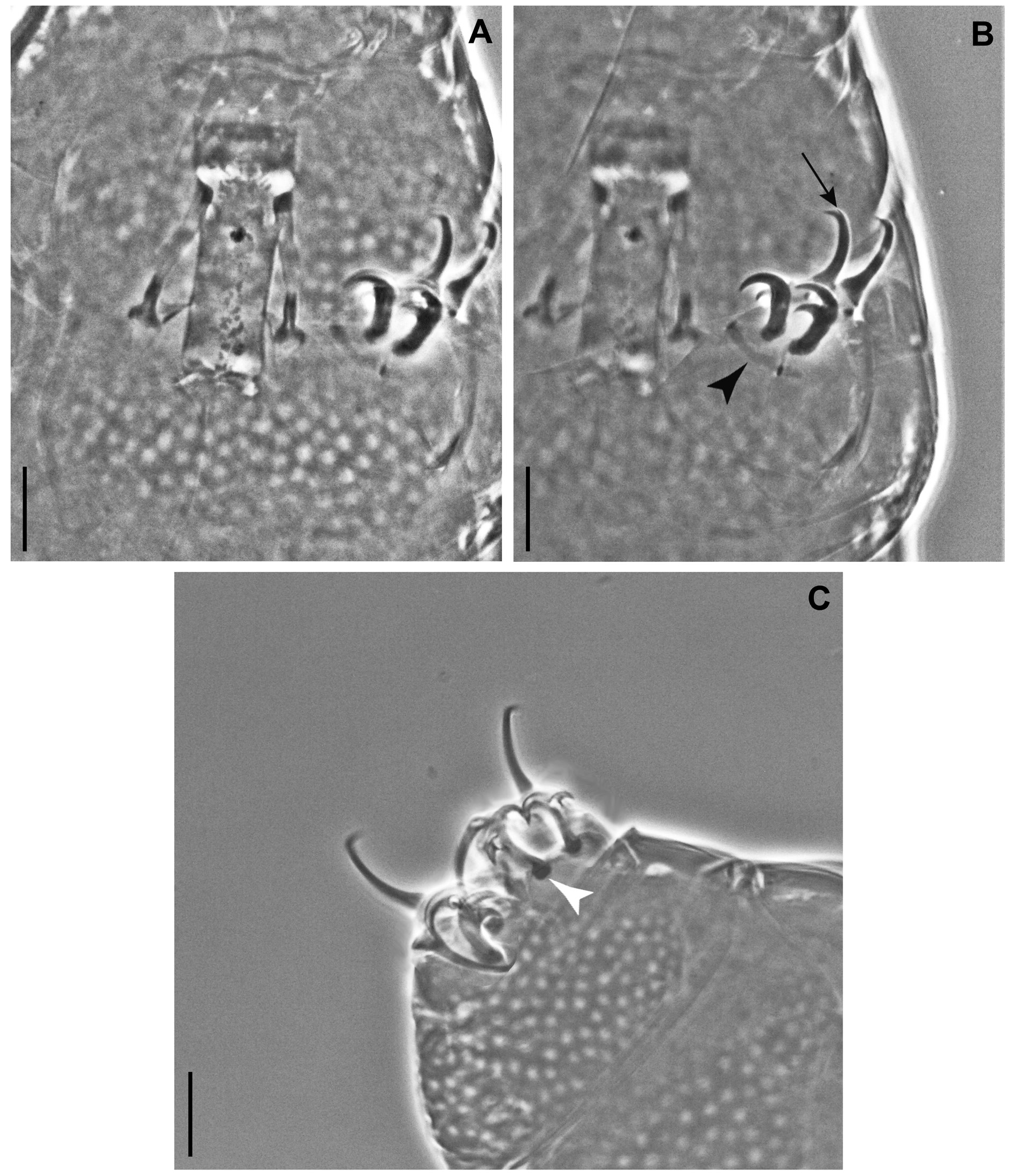
Claws of the Milnesium type with configuration [2-2]-[2-2] in young ( Fig. 7B–C View Fig ), [2-3]-[3-2] in senior specimens ( Fig. 12B–D View Fig ) but with very small basal spurs where present, in particular on legs IV where they are just a little spine ( Fig. 12B–D View Fig , white arrows); claws stout, secondary branches with basal thickenings (‘lunulae’; Figs 7C View Fig and 12C–D View Fig , white arrowheads), primary branches with small accessory points ( Figs 7B View Fig and 12B–C View Fig , black arrows); cuticular bars present on legs I–III ( Figs 7B View Fig and 12B–C View Fig , black arrowheads); percentual ratio of secondary branches with respect to primary branches for each claw couple higher for legs I, slightly lower for legs II–III and more significantly lower for legs IV ( Tables 1 View Table 1 and 2 View Table 2 ).
More detailed description is given in the following paragraphs separating young and senior specimens.
Young specimens (probably hatchlings: 216–305 µm; Figs 1 View Fig , 3–7 View Fig View Fig View Fig View Fig View Fig , Table 1 View Table 1 , Supp. file 1)
Cuticular dimples larger, especially in proportion to the body size (about 2–3.5 µm); dimples tend to form ten transverse bands almost touching one another and connected, at least in some areas, by dimples appearing less evident ( Fig. 1 View Fig ). Pseudoplates less developed and less distinct from one another in each row than is senior specimens ( Figs 4–5 View Fig View Fig ). Few simple, short, cuticular depressions can be present on some segments, tending to lay transversally, the most developed is constantly present at the level of legs III in the shape of a transverse groove ( Figs 1 View Fig , 3 View Fig , 6 View Fig , white arrowheads).
Buccal tube ( Fig. 7A View Fig ) more slender than in senior specimens (pt of standard width [43.7–52.1]).
Claw configuration [2-2]-[2-2] ( Fig. 7B–C View Fig ); percentual ratio of secondary branches with respect to primary branches for each claw couple with a less marked difference (than in senior specimens) between legs I and legs IV ( Table 1 View Table 1 ): 71–90% for claws I, 66–86% for claws IV, difference is 4–5%.
Senior specimens (probably from second instar on: 390–620 µm; Figs 1 View Fig , 8–12 View Fig View Fig View Fig View Fig View Fig , Table 2 View Table 2 , Supp. file 2) Cuticular dimples smaller than in young specimens (about 1–2 µm), especially in proportion to the body size; ten clear transverse bands of dimples, more spaced from one another than in young specimens and separated by smooth cuticle ( Figs 1 View Fig , 11 View Fig ). Starting with legs II in about 50% of the specimens, with legs III in the rest of them, irregular, usually branched cuticular depressions are present, tending to lay transversally or obliquely, more conspicuous on the caudal segments and all in general more developed and complex than in young specimens ( Figs 1 View Fig , 8 View Fig , 11 View Fig ).
Buccal tube ( Fig. 12A View Fig ) stouter than in young specimens (pt of standard width [55.2–64.0]). Claw configuration [2-3]-[3-2] ( Fig. 12B–D View Fig ); if these senior specimens include already the second instar, this would indicate early claw configuration change. Basal spurs of internal secondary branches I–III very small, and those of anterior secondary branches IV reduced to a little spine ( Fig. 12B–D View Fig , white arrows); secondary branches with basal thickenings (‘lunulae’) which are larger on legs IV ( Fig. 12C–D View Fig , white arrowheads); percentual ratio of secondary branches with respect to primary branches for each claw couple with a more marked difference between legs I and legs IV ( Table2 View Table 2 ): 87–99% for claws I, 72–86% for claws IV, difference is 13–15%.
Remarks
It was not possible to examine under SEM the (already mounted) studied material, therefore we considered the bright spots forming the reticular pattern visible under PCM as ‘dimples’(or depressions), and not true pseudopores (as defined by Morek et al. 2020a) as a consequence of an interpretation, also suggested by one anonymous reviewer. Such structures perfectly correspond in size, spatial distribution, and general appearance, to what can be seen in PCM images of many other species descriptions, in which the structures have been determined using SEM; true pseudopores are, instead, far smaller and usually more scattered and less visible. Milnesium pelufforum sp. nov. is the first species of the genus described with cuticular dimples that show some internal structure and form the reticulation arranged in ten transverse bands, as well as pseudoplates. The presence of ten transverse rows/bands of both structures, instead of a maximum of nine (the rule until now in Milnesium ) is the result of their presence in the new species also on the very first, cephalic, subsegment, where they were not found (or noticed) in the other species until now; this requires an update in the indication of the pseudoplate formula of all species of Milnesium with pseudoplates (see Discussion and Table 3 View Table 3 ). Morphometric data are given in Tables 1 View Table 1 (young specimens) and 2 (senior specimens); in Tables 4 View Table 4 and 5 View Table 5 (for senior and young specimens respectively) the statistically significant differences (through Student t -tests) of overlapping pt ranges of claw heights between the new species and the similar ones.
Differential diagnosis
Based on the presence of dimples on the dorsal cuticle, Milnesium pelufforum sp. nov. can be similar to many species of the old granulatum group ( Michalczyk et al. 2012a, 2012b), including both species with true dimples ascertained through SEM, and others in which the value of the bright spots visible under PCM (whether dimples or pseudopores) on the cuticle is still to be verified, but the appearance under PCM can be similar to the cuticular sculpturing of Milnesium pelufforum . The new species differs from all of them (indeed, from all congeneric species, according to their descriptions/redescriptions) by the presence of ten transverse bands of sculptured cuticle and rows of pseudoplates, and the presence of dimple internal structures.
We here provide separate differential diagnoses for young and senior specimens of the new species, due to the ontogenetic change, limiting the comparisons to species with the same claw configurations.
Senior specimens of Milnesium pelufforum sp. nov. can be similar to those of M. beasleyi Kaczmarek, Jakubowska & Michalczyk, 2012 , M. cassandrae Moreno-Talamantes, Roszkowska, García-Aranda, Flores-Maldonado & Kaczmarek, 2019 , M. krzysztofi Kaczmarek & Michalczyk, 2007 , M. lagniappe Meyer, Hinton & Dupré, 2013 , M. reticulatum Pilato, Binda & Lisi, 2002 and M. pacificum Sugiura, Minato, Matsumoto & Suzuki, 2020 by having a more or less similar cuticular ornamentation (seen under PCM) and claw configuration [2-3]-[3-2], but the senior specimens of the new species differ from them as follows:
1. Milnesium beasleyi only known from the type locality in Turkey, by different body colour: reddish in M. pelufforum sp. nov. vs yellow in M. beasleyi ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs no band arrangement described in M. beasleyi ; dimple diameter of about 1–2 µm in M. pelufforum vs 0.1–0.4 µm in M. beasleyi ; presence of pseudoplates in M. pelufforum vs not reported in M. beasleyi ; statistically significant lower pt of the lateral papillae, [13.8–19.9, mean 16.0] in M. pelufforum vs [19.6–23.7, mean 21.5] in M. beasleyi (t 11 = -10.06, p <0.001); different buccal tube width: higher pt of standard width [55.2–64.0] in M. pelufforum vs [31.2–39.8] in M. beasleyi ; statistically significant differences about pt of several claw heights ( Table 4 View Table 4 ).
2. Milnesium cassandrae (adults) only known from the terra typica ( Mexico; Moreno-Talamantes et al. 2019, 2020), by different body colour: reddish in M. pelufforum sp. nov. vs white or transparent with light yellow brownish tones before fixation in M. cassandrae ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs sparsely distributed, not forming bands or reticular design, in M. cassandrae ; presence of ten pseudoplate rows in M. pelufforum vs nine rows in M. cassandrae , and with different number in each row ( Table 3 View Table 3 ); statistically significant larger dimple diameter, 1–2 µm in M. pelufforum vs 0.6–1.4 µm in M.cassandrae (t 13 = -18.02, p <0.001); statistically significant higher pt of stylet support insertion point, [65.0–69.4, mean 67.0] in M.pelufforum vs [58.7–67.6, mean 63.5] in M. cassandrae (t 12 = 3.33, p <0.01); statistically significant higher buccal tube posterior/anterior width ratio, 91–100%, mean 97% in M. pelufforum vs 81–96%, mean 89% in M. cassandrae (t 12 = 4.39, p <0.001); statistically significant differences about pt of some claw heights ( Table 4 View Table 4 ).
3. Milnesium krzysztofi known from Costa Rica (type locality), Perú ( Kaczmarek et al. 2014) and Colombia ( Lisi et al. 2014; Londoño et al. 2015; Melo et al. 2015), by different body colour: reddish in M. pelufforum sp. nov. vs white or transparent in M. krzysztofi ; eyes present in M. pelufforum vs absent in M. krzysztofi ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs not forming bands in M. krysztofi ; different number of pseudoplates, not reported in M. krzysztofi , but visible in Kaczmarek & Michalczyk (2007: figs 2–7) in were it is possible to see at least six rows with different number/arrangement of pseudoplates; buccal tube nearly cylindrical in M. pelufforum vs funnelshaped in M.krzysztofi : anterior and posterior width of the buccal tube of this species not available from description but the difference is visually evident comparing Fig. 12A View Fig of the present paper with Kaczmarek & Michalczyk (2007: fig. 12); different buccal tube width: higher pt of standard width [55.2–64.0] in M. pelufforum vs [33.1–38.4], in M.krzysztofi ; statistically significant differences about pt of several claw heights ( Table 4 View Table 4 ).
4. Milnesium lagniappe only known from the type locality in USA, by different body colour: reddish in M. pelufforum sp. nov. vs white or transparent in M. lagniappe ; eyes visible in M. pelufforum vs not visible in M. lagniappe ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs arranged in nine bands in M. lagniappe ; different number/arrangement of pseudoplates, not reported in M. lagniappe , but visible in Meyer et al. (2013: fig. 1a-b) where it is possible to see at least seven rows different from the correspondent 10 rows of M. pelufforum ; statistically significant larger dimple diameter, 1–2 µm in M. pelufforum vs 0.7–1.3 µm in M. lagniappe (t 13 = 9,01, p <0.001); statistically significant lower pt of the peribuccal papillae, [19.5–25.9, mean 22.9] in M. pelufforum vs [22.7– 34.7, mean 28.0] in M. lagniappe (t 8 = 4.32, p <0.001); statistically significant lower pt of the lateral papillae:[13.8–19.9, mean 16.0] in M. pelufforum vs [16.9–30.5, mean 23.2] in M. lagniappe (t 11 = 3.79, p <0.001); lower pt of stylet support insertion point [65.0–69.4] in M. pelufforum vs [69.7–73.4] in M. lagniappe ; different buccal tube width: lower pt of standard width [55.2–64.0] in M. pelufforum vs [63.4–77.9] in M. lagniappe ; statistically significant differences about pt of several claw heights ( Table 4 View Table 4 ).
5. Milnesium reticulatum only known from the type locality in the Seychelles islands, by different body colour: reddish in M. pelufforum sp. nov. vs transparent in M. reticulatum ; different number of peribuccal lamellae: six in M. pelufforum vs four in M. reticulatum ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs nine in M. reticulatum ; presence of pseudoplates in M. pelufforum vs not reported in M. reticulatum ; higher pt buccal tube standard width, [55.2–64.0] in M. pelufforum vs [30.4–37.4] in M. reticulatum ; higher pt of many claw lengths: external primary and secondary branches of leg II, [46.7–55.2] and [37.7–47.6] in M. pelufforum vs [35.6–38.8] and [26.8–29.6] respectively in M. reticulatum ; internal primary and secondary branches II [43.6–52.2] and [37.6–45.0] in M. pelufforum vs [33.2–36.6] and [26.0–27.1] respectively in M. reticulatum ; external primary and secondary branches III [46.6–56.0] and [38.6–47.8] in M. pelufforum vs [35.6] and [26.8– 29.6] in respectively M. reticulatum ; internal primary and secondary branches of leg III [42.8–52.8] and [35.3–44.0] in M. pelufforum vs [33.2] and [26.0–27.1] respectively in M. reticulatum ; anterior primary and secondary branch IV [58.1–67.3] and [42.4–52.4] in M. pelufforum vs [37.9–39.7] and [29.2–33.0] respectively in M. reticulatum ; posterior primary and secondary branches branch IV [61.1–70.0] and [44.2–53.4] in M. pelufforum vs [41.7–44.3] and [29.2–35.0] respectively in M. reticulatum .
6. Milnesium pacificum only known from the terra typica in Japan, by different body colour: reddish in M. pelufforum sp. nov. vs creamy withe, transparent or with three brownish longitudinal stripes in M. pacificum ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs no band arrangement in M. pacificum ; dimple diameter about 1–2 µm in M. pelufforum vs about 0.50–0.65 µm in M. pacificum ; presence of ten rows of pseudoplates in M. pelufforum vs nine rows in M. pacificum ; statistically significant higher pt of the peribuccal papillae [19.5–25.9, mean 22.9] in M. pelufforum vs [16.3–22.4, mean 18.7] in M. pacificum (t 8 = 5.09, p <0.001); different buccal tube width: higher pt of standard width [55.2–64.0] in M. pelufforum vs [33.0–40.8] in M. pacificum ; statistically significant higher pt of stylet support insertion point [65.0–69.4, mean 67.0] in M. pelufforum vs [57.1–67.8, mean 62.2] in M. pacificum (t 12 = 4.54, p <0.001); statistically significant differences about pt of most claw heights ( Table 4 View Table 4 ).
Young specimens of Milnesium pelufforum sp. nov. can be similar to four species, M. cassandrae Moreno- Talamantes, Roszkowska, García-Aranda, Flores-Maldonado & Kaczmarek, 2019, M. katarzynae Kaczmarek, Michalczyk & Beasley, 2004 , M. pacificum Sugiura, Minato, Matsumoto & Suzuki, 2020 and M. variefidum Morek, Gąsiorek, Stec, Blagden & Michalczyk, 2016 , due to a more or less similar cuticular ornamentation and claw configuration [2-2]-[2-2]. Young specimens of M. pelufforum differ from them as follows:
1. Milnesium cassandrae (hatchlings and youngs) only known from the terra typica ( Mexico; Moreno- Talamantes et al. 2019, 2020), by different body colour: reddish in M. pelufforum nov. sp. vs white or transparent with light yellow brownish tones before fixation in M. cassandrae ; presence of ten rows of pseudoplates, better developed, in M. pelufforum vs nine rows poorly developed in M. cassandrae ; eyes present in M. pelufforum vs absent in hatchling and youngs of M. cassandrae ; different buccal tube width: higher pt of standard width [43.7–52.1] in M. pelufforum vs [28.2–39.4] in M. cassandrae ; statistically significant higher pt of stylet support insertion point [65.3–73.8, mean 71.6] in M. pelufforum vs [56.5–70.5, mean 66.3] in M. cassandrae (t 12 = 4.38, p <0.005); statistically significant differences about pt of almost all claw heights ( Table 5 View Table 5 ).
2. Milnesium katarzynae known from China, Sichuan Province (type locality), Costa Rica ( Kaczmarek et al. 2014) and Colombia ( Caicedo et al. 2014; Londoño et al. 2015; Melo et al. 2015), by different body colour: reddish in M. pelufforum sp. nov. vs white in M. katarzynae ; eyes present in M. pelufforum vs absent of M. katarzynae ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs no band arrangement in M. katarzynae ; dimple diameter of about 2–3.5 µm in young specimens of M. pelufforum vs about 0.5–1.5 µm in M. katarzynae ; pseudoplates present in M. pelufforum vs not reported in M. katarzynae ; different buccal tube width: higher pt of standard width [43.7–52.1] in M. pelufforum vs [21.7– 26.6] in M. katarzynae ; statistically significant higher pt of stylet support insertion point [65.3–73.8, mean 71.6] in M. pelufforum vs [73.3–78.3, mean 75.8] in M. katarzynae (t 12 = -6.13, p <0.001); different pt of external primary and secondary branches I, [41.8–48.9] and [37.4– 46.2] in M. pelufforum vs [40.0–40.7] and [26.6–26.7] respectively in M. katarzynae ; external primary and secondary branches II [46.7–55.2] and [37.7–47.6] vs [40.0–40.7] and [26.7–28.3] respectively in M. katarzynae ; external primary and secondary branches III [46.6–56.0] and [38.6–47.8] vs [40.7–41.8] and [28.3] respectively in M. katarzynae , anterior primary and secondary branches IV [58.1–67.3] and [42.4–52.4] vs [43.5–43.8] and [26.7–28.3] respectively in M. katarzynae .
Level along the body Anterior Leg I Between I–II Leg II Between II–III Leg III Behind leg III Posterior Reference Rows (present paper) I II III IV V VI VII VIII IX X Rows ( Moreno-Talamantes et al. 2019) (I) (II) (III) (IV) (V) (VI) (VII) (VIII) (IX) M. pelufforum sp. nov. Present paper: formula Figs 5, 10 (schematic drawings) Figs 4, 9 (UV fluorescence) 1 4 6 6 10 10 10 12 12 4 M. irenae sp. nov. Present paper: formula Fig. 15 (schematic drawing) Fig. 14 (UV fluorescence) – 4 4 8 10 8 10 8 12 4 M almatyense Morek et al. 2020b Morek et al. 2020b: Fig. 2a (PCM) Fig. 2b (schematic drawing) Fig. 3a–d, e (SEM) –?? 4 4 2 4 4 10 1 M. berladnicorum Morek et al. 2016 Morek et al. 2016: Fig. 6a (schematic drawing) Description – 1? 1? 2? 2 2 6 4 8 2 M. cassandrae Moreno-Talamantes et al. 2019 Moreno-Talamantes et al. 2019: formula Fig. 1a (PCM) Fig. 3 (UV fluorescence) Fig. 4 (schematic drawing) – 4 2 4 10 6 10 8 10 2 M eurystomun Morek et al. 2020a Morek et al. 2020a: Fig. 8 (PCM) Fig. 9 (schematic drawing) Description – 2 2 2 4 2 4 4 10 4 M variefidum Morek et al. 2016 Morek et al. 2016: Fig. 2a (PCM) Fig. 2b–c (SEM) Fig. 2d (schematic drawing) Description
– – – 2 6 2 6 4 10 2
3. Milnesium pacificum (hatchlings) only known from the terra typica in Japan, by different body colour: reddish in M. pelufforum sp. nov. vs creamy white, transparent or with three brownish longitudinal stripes in M. pacificum ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs no band arrangement in M. pacificum ; dimple diameter about 2–3.5 µm in M. pelufforum vs about 0.61– 0.82 µm in M. pacificum ; shorter peribuccal papillae, pt [14.7–15.1] in M. pelufforum vs [15.8–20.7] in M. pacificum ; longer lateral papillae, pt [14.6–15.9] in M. pelufforum sp. nov. vs [12.1–14.2] in M. pacificum ; different buccal tube width: higher pt of standard width [43.7–52.1] in M. pelufforum vs [30.1–39.4] in M. pacificum ; statistically significant higher pt of stylet support insertion point [65.3– 73.8, mean 71.6] in M. pelufforum vs [58.7–69.2, mean 63.9] in M. pacificum (t 12 = 5.18 p <0.001); statistically significant differences about pt of many claw heights ( Table 5 View Table 5 ).
4. Milnesium variefidum (hatchlings and youngs) only known from the type locality in Scotland, by the body colour: reddish in M. pelufforum sp. nov. vs yellowish before fixation and transparent afterwards in M. variefidum ; sculptured cuticle with dimples arranged in ten bands in M. pelufforum vs cuticle appearing smooth but with faint pseudopores, not arranged in bands, in M. variefidum ; presence of ten pseudoplate rows better visible and outlined in M. pelufforum vs seven rows of pseudoplates only occasionally visible or poorly developed in M. variefidum ( Table 3 View Table 3 ); different buccal tube width: pt of standard width [43.7–52.1] in M. pelufforum vs [22.1–33.8] in M. variefidum ; different pt ranges of claw heights: external primary and secondary branch I [45.0–53.7] and [37.9–47.4] in M. pelufforum vs [33.7–44.7] and [27.7–35.8] in M. variefidum respectively; internal secondary branch I [35.9–44.7] in M. pelufforum vs [28.2–35.4] in M. variefidum ; internal primary branch II and III [46.0–56.5] and [47.9–57.9] in M. pelufforum vs [33.9–45.6] and [34.2–47.2] in M. variefidum respectively; statistically significant differences about pt of most of the other claw heights ( Table 5 View Table 5 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |