Saguinus fuscicollis (Spix, 1823)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730870 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFC3-FFD2-FAD8-FB61664DEA65 |
|
treatment provided by |
Conny |
|
scientific name |
Saguinus fuscicollis |
| status |
|
31 View On .
Spix’s Saddle-back Tamarin
Saguinus fuscicollis View in CoL
French: Tamarin de Spix / German: Braunriickentamarin / Spanish: Tamarin de Spix Other common names: Brown-mantled Tamarin, Hershkovitz's Saddle-back Tamarin, Saddleback Tamarin; Acre Saddle-back Tamarin (acrensis), Avila-Pires’s Saddle-back Tamarin (avilapiresi), Cruz Lima's Saddle-back Tamarin (cruzlimai), Gray-fronted Saddle-back Tamarin (mura), Lako's Saddle-back Tamarin (primitivus)
Taxonomy. Midas fuscicollis Spix, 1823 View in CoL ,
Brazil, near Sao Paulo de Olivenca, between Rio Solimoes and Rio Ica, Amazonas. Restricted by P. Hershkovitz in 1977 to near Sao Paulo de Olivenca but to the south of the Rio Solimoes.
In his major synthesis of 1977, P. Hershkovitz placed all the 14 of the saddle-back tamarins he recognized as subspecies of S. fuscicollis . One of them, S. f. acrensis , described by C. Carvalho in 1957, was found to be a hybrid S. f. fuscicollis x S. weddelli melanoleucus. Molecular genetic analysis showed that the form fuscus was more closely related to S. nigricollis than to S. fuscicollis and argued that it should be considered a separate species. Further research on S. fuscus and S. nigricollis is needed to resolve the taxonomy and phylogeny of the Black-mantled and saddle-back tamarins in northern Peru, Colombia, and Ecuador. The taxonomy suggested by the molecular genetic analysis of C. Matauschek and coworkers in 2011 is followed here; they considered the former subspecies lagonotus , tripartitus , nigrifrons , and weddelli to be distinct species. The Central Amazonian Brazilian subspecies have yet to receive similar molecular genetic analyses and remain, by default, as subspecies of S. fuscicollis . Five subspecies recognized.
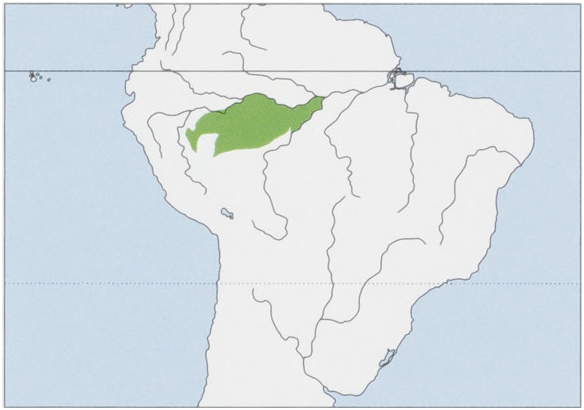
Subspecies and Distribution. S. J. fuscicollis Spix, 1823 — W Brazil (states of Acre & Amazonas) and Peru, S of the Rio Solimoes, between the Rio Javari in the W, E through the Rio Jutai Basin to the Rio Jurua (left bank), also in Peru, W of the Rio Yavari as far as the Rio Tapiche, an E tributary of the Rio Ucayali, and extending N from there as far as the Rio Blanco (left bank), where it meets the distribution of Geoffroy’s Saddle-back Tamarin, S. nigrifrons (right bank of the Rio Blanco). S. f. auvilapiresi Hershkovitz, 1966 — W Brazil in the Amazonas State (type locality is the mouth of the Lago de Tefé, Rio Solimoes), found along the S of the Rio Solimoes between the rios Jurua and Purus, including the basins of the rios Urucu and Coari, and probably the Rio Tefé; the S limits are not known but possibly in the region of the N bank of the Rio Tapaua, an affluent of the Rio Purus. S. f. eruzlimai Hershkovitz, 1966 — W Brazil, described by Hershkovitz without provenance (based on a single individual “said to be from the upper Rio Purus”); M. G. M. van Roosmalen reported in 2003 that it had been observed by T. van Roosmalen on 21 June 2002, on the W bank of the Rio Purus, opposite the mouth of the Rio Sepatini, and noted that Hershkovitz was correct in supposing that it occurred between the rios Pauini and Tapaua, W of the Rio Purus. S. J. mura Rohe et al, 2009 — C Brazil (Amazonas State), interfluvium of the rios Madeira and Purus, S of the Rio Amazonas, probably S to the Rio Igapo-Acu. S. f. primitivus Hershkovitz, 1977 — W Brazil (Amazonas State), the distribution is believed to extend from the left bank of the Rio Pauini, along the left bank of the upper Rio Purus, N to the Rio Tapaua (right bank), as far W the right bank of the Rio Jurua, and the Rio Tarauaca. View Figure
Descriptive notes. Head—body c.21 cm,tail ¢.32 cm; weight mean 333 g (n = 23). Spix’s Saddle-back Tamarin is a small, highly variable species noted for its marbled or mottled saddle. The head, arms, legs, and forepart and hindpart of the back are solid or ticked black, brown, or red. The tail is reddish or brownish at the base (at least on the underside), with the remainder being black or agouti. The forehead and crown are blackish or agouti, and the nape and mantle are usually agouti. The face is hairy, with black skin, and ears are large and bare. In the nominate form fuscicollis , the crown, forehead, and temples are dominantly orange-agouti. Cheeks are black, and the mantle is a dark agouti to blackish brown, extending only to a little beyond the shoulders. Facialskin is black, with the short grayish-white hairs surrounding the mouth and nostrils. Sides of the face are blackish, giving the appearance of a black mask across the eyes. Arms are the same color as the mantle. The middle and lower back is marbled black, with buffy or orange hairs. The rump and thighs are reddish, and upper surfaces of hands and feet are black. The throat, neck, and chest are dark or reddish brown, mixed with black hairs. The tail is black, except a short basal part is rufous. External genitalia are mostly or entirely pigmented black. “Avila-Pires’s Saddle-back Tamarin” (S. f. avilapiresi ) is the most somber and uniformly colored of the subspecies. Its body is predominantly a dark brown throughout, and the forehead, crown, mantle, and upper arms are blackish brown and finely ticked with orange hairs. The rump and thighs are blackish brown, like the mantle—a key distinguishing feature. Lower arms are blacker. Facial skin is black. Hairs around the mouth are pale gray, a little longer than is usual for fuscicollis , and form a triangular patch on each side of the muzzle with the apex beneath the eyes. The saddle on the middle of the back is blackish brown, with the fringe on the sides of the back a little buffier in color. Lower legs and inner sides of legs are darker than the thighs. Upper surface of hands and feet are black. The throat, neck, chest, and belly are brown and finely ticked with orange. The tail is black, except for the base which is brown like the rump. External genitalia are pigmented black. Diagnostic features for “Cruz Lima’s Saddle-back Tamarin” (S. f. cruzlimai ) include the reddish orange of the crown, mantle, arms, and legs. The forehead has a well-defined whitish transverse band (chevron). It is distinguished from the two subspecies of Weddell’s Saddle-back Tamarin (S. weddelli weddelli and S. w. crandalli) by the reddish-orange forequarters and outer thighs. Facial skin is black, and, as in all Spix’s Saddle-back Tamarin, there are grayish white hairs around the mouth and nostrils. Hands and feet are pale gray. The tail is blackish. In the “Gray-fronted Saddleback Tamarin” (S. f. mura ), the forehead is mostly dark brown but with sparse grayish hairs. It lacks the white chevron above the eyebrows. The saddle is strongly mottled ocherous with dark brown to black, beginning on the scapular region and extending to the dorsal base of tail. Arms are black and ticked with reddish brown on the lateral surfaces. In some individuals, there are white hairs in small numbers forming a faint line on each side of the lowest part of the gular region where it meets the upper chest. In “Lako’s Saddle-back Tamarin” (S. f. primitivus ), the crown, mantle, arms, legs, rump, and chest are agouti-colored. There is a well-defined grayish transverse band along the forehead, separated from the crown by a narrow blackish line. The saddle is weakly defined from the mantle, with hairs there having 1-3 reddish bands. The chin is gray, and the throat and neck are predominantly blackish. Upper surface of hands and feet are a dark agouti. The tail is agouti-colored at the base and blackish distally.
Habitat. P. Soini studied some aspects of the ecology and demography of the nominate form fuscicollis along the right bank of the Rio Tapiche, Peru, and Avila-Pires’s Saddle-back Tamarin has been studied in the wild on the Rio Urucu, Brazil, by C. A. Peres. Some observations of Avila-Pires’s Saddle-back Tamarin were also made by A. Johns on the west bank of Rio Tefé, Brazil, nearits type locality. Avila-Pires’s Saddleback Tamarin occurs in inundated forest (igapo) and palm swamps, creekside forest, windfall areas, and dense vegetation in tall terra firma forest. At Tefe, Avila-Pires’s Saddle-back Tamarin was scarce, occurring in small groups of 2-3 individuals and only in the mosaic of secondary growth and cultivation. The sympatric Mustached Tamarin (8. mystax ), in contrast, was common, occurring in groups of up to 15 individuals in tall primary forest and logged forest as well as a forested island, besides the cultivated mosaic. Spix’s Saddle-back Tamarins spend most of their time in the understory of the forest up to 10 m above the ground, although they will go up to the canopyto feed at 30-40 m. Structural differences in the understory where they forage for animal prey probably explain their absence in forest types occupied by Mustached Tamarins, which are typically foliage-gleaning predators in lower and middle canopies above them.
Food and Feeding. Diets of Spix’s Saddle-back Tamarins include animal prey; ripe fruits (mainly small, soft, and yellow or red mesocarps and whole berries) of trees,lianas, and epiphytes; nectar and exudates such as gums (from tree-trunks, woody lianas, and seed pods ofthe tall leguminous emergents Parkia nitidaand P. pendula, Fabaceae ); and on very rare occasions unripe seeds and fungi. During one year, Spix’s Saddle-back Tamarins fed on 180 plant species in 43 plant families (especially Moraceae , Fabaceae, Guttiferae , Linaceae and Sapotaceae )—an extremely diverse diet. Depending on the season, as many as 46 plant species were used (mid-late wet season) or as few as 18 (late dry season) during each month. Each day, they exploited as few as five species of food trees during the peak dry season but as many as 24 species in times of fruit abundance in the wet season. Gums and nectar from the red flowers of Symphonia globulifera (Guttiferae) were particularly important during the dry season when fruit was scarce. Overall, gums from Parkia seed pods were the single most food item, accounting for c.10% of annual feeding time. Readily available gums from oozing bark of Vochysia guianensis ( Vochysiaceae ) and the palm Jessenia bataua ( Arecaceae ) were also eaten. Mixed groups of Avila-Pires’s Saddle-back Tamarins and Mustached Tamarin (subspecies pileatus) travel together throughout the day and coordinate their use of food patches. They differ in their animal-prey foraging methods. Avila-Pires’s Saddle-back Tamarins forage mainly in closed substrates, investigating the natural shelters of cryptic prey, plucking them from under bark, from crevices in woody lianas,trees, tangles of vines or epiphytes, and from rosettes of bromeliads and suspended leaflitter. They are manipulative foragers, seeking concealed food items. About one-half of the prey items is caught this way, but a large number are also obtained from those displaced from the mid-story foliage by Mustached Tamarins foraging above; Peres estimated that 66-73% of their prey biomass was obtained from flushed items. This fosters the association of saddle-back tamarins with Mustached Tamarins. In general, saddle-back tamarins rarely locate and capture mobile prey exposed on leaf surfaces, which is typical for Mustached Tamarins. Avila-Pires’s Saddle-back Tamarins concentrate on larger prey than do Mustached Tamarins. Items are mainly arthropods but also occasionally small vertebrates such as Anolis lizards. Insects eaten are mainly orthopterans, including katydids ( Tettigoniidae ), praying mantis ( Mantidae ), and short-horned grasshoppers ( Acrididae ). They also eat stick insects ( Phasmidae ), cockroaches ( Blattidae ), spiders, scale insects, scorpions, cuckoo-spit aphids ( Aphrophoridae , Hemiptera ), and larva of social insects, including ants, termites, and even wasps.
Breeding. During the year-long study at Urucu, births of Spix’s Saddle-back Tamarins and Mustached Tamarins were not synchronized. The larger Mustached Tamarin has a longer gestation and takes longer to wean its infants. In 14 sets of twins recorded for both species, ten litters were born in late June to early October (early dry season) and four litters in December—January (early wet season), demonstrating that, at least in some groups, births may occur twice a year.
Activity patterns. Avila-Pires’s Saddle-back Tamarins were active for about nine hours each day. They started their day at sunrise and returned to their sleeping sites in the late afternoon at 16:00-17:00 h. One-third of their time was spent moving, and onefifth was spent resting. Foraging for, manipulating, and eating animal prey occupied 43% of their day. The remainder of their time was spent eating fruits and plant exudates, engaging in social activities such as allogrooming, resting, in territorial activities, mobbing prey, and alarm calling. Mustached Tamarins and saddle-back tamarins that travel together through much of the day split company when they retire to their sleeping sites, usually between 15 m and 120 m apart. Avila-Pires’s Saddle-back Tamarins almost always used tree holes as sleeping sites, whereas Mustached Tamarins slept mostly in clumps of dense vegetation, such as large liana festoons and vine tangles.
Movements, Home range and Social organization. Avila-Pires’s Saddle-back Tamarins form stable groups of 5-8 individuals, always associated with somewhat larger groups of 8-11 Mustached Tamarins during the day. Distances traveled during the day vary from 1150 m to 2700 m, with an average of 1991 m. At Urucu, home ranges of 145 ha were the largest recorded for the genus. Density at Urucu was very low as a result at 1-8 groups/km? or 6-8 ind/km?* and below the lowest values reported for any site where mixed-species groups of tamarins have been studied. Density of Avila-Pires’s Saddle-back Tamarins was 8-9 ind/km® (mean group size of five) at Igarapé Acu, downstream on the left bank of the Rio Urucu, and 10 ind/km?* (mean group size of four) at another site on the right bank of the Rio Tefé. These low densities have been attributed to a lack of heterogeneity of successional and climax forest, along with nutrient-poorsoils, strong seasonality in ripe fruit production, lack of successional forest, and a weak staggering of fruiting peaks among different habitats. Densities of the nominate subspecies fuscicollis at threesites varied from 14 ind/km* (mean group size of 3-7) to 27 ind/km? (mean group size of 5-6). At the Urucu site, one mixedspecies group of Avila-Pires’s Saddle-back Tamarins and Mustached Tamarins suffered attacks from raptors about once every nine days. Unsuccessful attacks included those by ornate hawk-eagle (Spizaetus ornatus). Nine other raptors that occur in the forest there are known or potential predators of these small primates : harpy eagle (Harpia harpyja), Guiana crested eagle (Morphnus guianensis), black-and-white hawk eagle (Spizastur melanoleucus), and four species of hawks and buzzards (Leucopternis, Buteo, and Buteogallus).
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List. The subspecies primitivus is classified as Data Deficient, and the subspecies mura has not yet been assessed. Serra do Divisor National and Jutai-Solimoes Ecological Station are within the distribution of the nominate subspecies fuscicollis . Lago Ayapua State Environment Protection Area and Abufari Biological Reserve on the left bank of the Rio Purus are within the distribution of avilapires:.
Bibliography. Aquino & Encarnacion (1994b), Cheverud (1995), Cheverud & Moore (1990), Cheverud et al. (1993), Cropp et al. (1999), Epple & Katz (1980, 1984), Haugaasen & Peres (2005a), Hershkovitz (1966, 1977), Hodun et al. (1981), Johns (198ba, 1986¢, 1991), Matauschek et al. (2011), Peres (1991a, 1992a, 1992b, 1993b, 1993c, 1993d, 1996b, 2000a, 2000b), Peres et al. (1996), Réhe et al. (2009), Snowdon & de Soini (1988), Soini (1990a, 1990b), Soini & Céppula (1981), Soini & Soini (1983, 1986).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.