Saguinus weddelli (Deville, 1849)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730872 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFC2-FFDC-FA09-F5B46729E819 |
|
treatment provided by |
Conny |
|
scientific name |
Saguinus weddelli |
| status |
|
32 View On .
Weddell’s Saddle-back Tamarin
French: Tamarin de Weddell / German: Schwarzmanteltamarin / Spanish: Tamarin de Weddell Other common names: Crandall's Saddle-back Tamarin (crandalli), White-mantled / White Saddle-back Tamarin (melanoleucus)
Taxonomy. Midas weddelli Deville, 1849 ,
Bolivia, province of Apolobamba (= Caupolican), La Paz, Bolivia .
Formerly considered a subspecies of S. fuscicollis . A recent molecular genetic analysis placed it in a distinct clade with the forms fuscicollis , nigrifrons , and melanoleucus, along with leucogenys specimens sampled south of the Rio Pachitea. The form melanoleucus , also formerly a subspecies of S. fuscicollis , was considered a distinct species in the 1990s, with crandalli, a pale form of unknown provenance, as a subspecies. Molecular genetic analyses have found that differences between melanoleucus and weddelli were no larger than among the weddelli specimens, but because ofits distinctive pelage (white) and defined geographic distribution, melanoleucus is maintained as a subspecies of S. weddelli . The “Acre Saddle-back Tamarin” (S. fuscicollis acrensis) from the upper Rio Jurua is a hybrid S. fuscicollis x S. w. melanoleucus. “Crandall’s Saddle-back Tamarin” (c¢randalli) is known only from a single specimen and is listed here as a subspecies of S. weddelli , but it may be a hybrid. Three subspecies recognized.
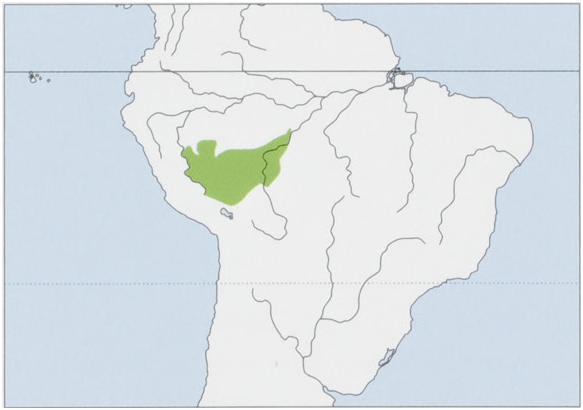
Subspecies and Distribution. S. w. weddelli Deville, 1849 — W Brazil (between the rios Purus and Madeira in the states of Amazonas, Acre, and NW Rondonia, as far N as the Rio Pixuna) to SE Peru (from the Rio Abujao, E tributary of the Rio Ucayali, to the S along both banks of the Rio Ucayali, E of the Andes, E of the Rio Apurimac, and along the upper reaches of the Apurimac, Inambari, Urubamba, and Tambopata), and to N Bolivia (rios Madeira and Beni or Mamoré); it crosses the upper Rio Madeira to its right bank in Rondonia in the region of the Rio Jamari,S of the Rio Ji-parana, being sympatric there with Rondon’s Marmoset ( Mico rondonz). S. w. crandalli Hershkovitz, 1966 — provenance unknown but possibly near the headwaters of the rios Jurua and Tarauaca in W Brazil. S. w. melanoleucus Miranda Ribeiro, 1912 — Brazilian Amazon, along the right bank of the upper Rio Jurua, S from the mouth of the Rio Eira, up to its headwaters, E to the left bank of the Rio Tarauaca (no saddle-back tamarins have been recorded to the E of the Rio Tarauaca in Acre State as far as the upper Rio Purus), in SE Peru from the upper reaches of the Rio Breu and the Quebrada Breu, right bank affluents of the upper Rio Yurua. View Figure
Descriptive notes. Head-body 18-27 cm, tail 25-38 cm; weight 340-440 g. In the nominate form weddelli , the crown, sides of head, throat, neck, chest, upper surfaces of hands and feet, mantle, and arms are black or blackish brown. The mantle often has sparse orange ticking. The forehead has a distinctive white transverse band (chevron). Facial skin is pigmented black, and there are short gray hairs around the mouth and nostrils. The saddle is vermiculated or marbled black, with ocherous buff or gray tones. The rump and thighs are reddish orange. Innersides of forearms are black, variably mixed with orange or reddish. Inner sides of upper arms are black to dominantly reddish. The belly is reddish orange. The tail is black, except for a short rufous basal part. External genitalia are mostly or entirely pigmented. The “White-mantled Saddleback Tamarin” (S. w. melanoleucus) is almost entirely creamy white (often washed or streaked with yellowish buffy hairs), contrasting the black of the ears, facial skin, and external genitalia. Underparts and inner surface of limbs are whitish or yellowish to ocherous. The forehead has a poorly defined white band, and hairs surrounding the mouth and nostrils are grayish. In the “Crandall’s Saddle-back Tamarin” (S. w. crandally), the crown, mantle, and arms are yellowish drab. The rump, legs and thighs are orange, and the tail is brown with orange underneath on the proximal one-fifth. There is a broad, well-defined, white, transverse band across the forehead (chevron). The throat, neck, and chest are buffy to nearly white. Facial skin is pigmented. There are short gray hairs around the mouth and sides of the nostrils. The saddle is vermiculated buffy and black. Upper surfaces of hands are drab, and those of the feet are mixed buffy and orange, with dark bases of the hairs showing through. External genitalia are pigmented black.
Habitat. In Bolivia, the nominate form weddelli can be found in tall forest with sparse understories (“monte alto”), tall forest without a closed canopy and dense understories (“monte bajo”), secondary forests with a canopy heights of 10-20 m (“barbecho claro”), and dense secondary forests without a closed canopy (“barbecho tupido”). It shows a preference for secondary forest; in one study in Bolivia, ¢.70% of their day was spent in such forest. In Manu National Park and Biosphere Reserve, Peru, home ranges always contain a mosaic of forest types and always with successional forest patches from windfalls or new growth from changes in river courses. They do not occur in large tracts of mature forest with sparse understories. A comparative study of habitat use by Weddell’s Saddle-back Tamarin showed that they tended to use small (less than 5 cm in diameter), medium, and large branches (more than 10 cm in diameter) in roughly equal proportions, whereas “mustached tamarins” (Red-bellied Tamarin, S. labiatus , and Emperor Tamarin, S. imperator ) used small supports more and larger supports less. Weddell’s Saddle-back Tamarins used horizontal supports much less and vertical supports much more, and spent much more time on tree trunks and less time on branches than Red-bellied and the Emperor tamarins. When jumping, they went from branch to branch much less and from a branch to a trunk, or between trunks, more than the two species of mustached tamarins, which mostly jumped from branch to branch. Weddell’s Saddle-back Tamarins foraged for animal prey at an average height above the ground of 6-1 m and forfruits at 11-2 m. In contrast, the two species of mustached tamarins foraged for insects at an average of 9-4 m above the ground and for fruits at 16-7 m. These measurements clearly define structural and vertical differences in forest habitats occupied by these species and differences in foraging techniques and sites, resulting in clear differences in their animal prey. Demographic and ecological studies of Weddell’s Saddle-back Tamarin living in mixed groups with the Emperor Tamarin were carried out by J. Terborgh and A. Goldizen for more than 13 years at Cocha Cashu in Manu National Park and Biosphere Reserve, Peru. J. Crandlemire-Sacco did a 15-month comparative study of Weddell’s Saddle-back Tamarins with titi monkeys (Callicebus) in the Tambopata National Reserve on the Rio Madre de Dios in southern Peru. Other studies have focused on ecology, behavior, and polyspecific associations of Weddell’s Saddle-back Tamarin with Red-bellied Tamarin and Goeldi’s Monkey ( Callimico goeldii ) in the Pando region in north-western Bolivia. They began with a five-month study and survey by A. and G. Pook in 1978-1979 and were followed by M. Yoneda who carried out a six-month study in 1979, H. Buchanan-Smith who carried out field studies and surveys at a number of sites during five months in 1987 and again in 1997, and S. Hardie who studied them during six monthsat two sites in 1991. L. Porter has been studying polyspecific groups of tamarins and Goeldi’s Monkey at a site just north of the Rio Tahuamanu in the Pando region since 1998. In Brazil, M. A. Lopes and S. F. Ferrari carried out a nine-month field study of sympatric Rondon’s Marmosets and Weddell’s Saddle-back Tamarins at the Samuel Ecological Station on the Rio Jamari, Rondonia State,].-C. Bicca-Marques studied cognitive aspects of foraging in mixed-species groups of Weddell’s Saddle-back Tamarins and Emperor Tamarins at the 100-ha Zoobotanical Park of the Federal University of Acre in 1997-1998, and J. Rehg studied mixed-species groups with Emperor Tamarins and Goeldi’s Monkeys near Rio Branco, Acre State, in 1999-2003.
Food and Feeding. At Manu, the nominate subspecies weddelli forms mixed-species groups with the “Bearded Emperor Tamarin” (S. imperator subgrisescens), where they have c¢.43% overlap in the fruits they eat. A large number of fruiting trees are exploited at any one time, but only a few are visited on any one day. Likewise, fruits from a large number of species are included in the diet over the year, but only six or seven constitute key resources, providing the large majority of the tamarins’ carbohydrate requirements. Exploited fruit crops typically ripen in a piecemeal fashion over long of periods of time. The vine Celtis iguanaea ( Ulmaceae ), for example, is an important source of fruits (drupes) for tamarins in April-July. Fruits are not eaten by larger primates such as capuchins ( Cebus and Sapajus ) or squirrel monkeys ( Saimiri ). Celtis fruits, nectar from flowers of the vine Combretum assimile ( Combretaceae ), and nectar and berries of Quararibea cordata trees ( Bombacaceae ) are key food sources for both species of tamarins during the dry season. These food sources are too spread out in the forest and provide quantities of food that are too small for any systematic exploitation by other primate species. Even so, the two species of tamarins lost weight at this time of relative fruit scarcity. In the case of Combretum , systematic visitation to their inflorescences implies that tamarins are important pollinators. The main difference in the diets of Weddell’s Saddle-back Tamarins and Emperor Tamarins is in their methods and localities for searching for animal prey. Weddell’s Saddle-back Tamarins dedicated 62% of their foraging time to investigating knotholes and crevicesin trees, vines, and palms for hidden prey, 22% looking along branches, and 8% looking among leaves. In contrast, Emperor Tamarins were scanning foliage-gleaners (86% of their foraging time) and used a stealth-and-pounce capture method for exposed but often cryptic prey; less than 1% of their foraging was spent investigating crevices. Emperor Tamarins spent more than 50% of their time foraging among vines, whereas Weddell’s Saddleback Tamarins searched mostly lower down on tree trunks (68%). The two species exploited different animal prey. The nominate form of Weddell’s Saddle-back Tamarin caught more large insects (e.g. grasshoppers, Orthoptera ) than Emperor Tamarins, which, while also showing a definite preference for grasshoppers, consumed many more butterflies and moths ( Lepidoptera ), especially their larvae and pupae. Weddell’s Saddle-back Tamarin also eats more vertebrate prey, mostly lizards that they seize from tree trunks. In the Pando region of northern Bolivia,it also forms mixed groups with the nominate form of the Red-bellied Tamarin (S. labiatus labiatus ). There, they feed on gums, fruits, and nectar from 33 plant species. Fruits of Pourouma and Cecropia sciadophylla (both Urticaceae ) and the nectar of Symphonia globulifera (Guttiferae) were particularly important. Differences between the two tamarins in their animal foraging method, and the prey taken, were similar to those found in Manu National Park and Biosphere Reserve where Weddell’s Saddle-back Tamarins associated with the Emperor Tamarin. In the Pando region of northern Bolivia, Weddell’s Saddle-back Tamarins form associations not only with the Red-bellied Tamarin (subspecies labiatus ) but also on many occasions with Goeldi’s Monkeys. Groups of nine weddelli , five labiatus , and eight Goeldi’s Monkeys shared a home range of 30-35 ha during three months. The association, usually established in the early mornings by long calling of the groups, was most evident when they rested; weddelli and labiatus spent an average of ¢.50% of their day together, and sometimes they were together for the entire day. Goeldi’s Monkey associated with one or both of the tamarins for ¢.50% of its time, but it was more frequently observed with weddelli than labiatus . When traveling, Redbellied Tamarins tended to lead and were followed by the saddle-backs and then the Goeldi’s Monkeys. Red-bellied Tamarins moved higher up in the forest (77% of their traveling and foraging above 10 m from the ground) and traveled more quickly, using mainly quadrupedal running and jumping. Goeldi’s Monkeys often lagged behind by as much as ten minutes, using their predominantly vertical-clinging and leaping locomotion in the lowest part of the understory below 5 m above the ground. Weddell’s Saddle-back Tamarins moved and foraged above them, usually below 10 m above the ground, using a mix of vertical-clinging and leaping and quadrupedal running and jumping. The mustached tamarins also tended to be the first to begin activity after a resting period. As was found in Manu, the larger mustached tamarins were dominant to the smaller saddle-backs when the two interacted socially or had a dispute (rarely) over a food item.
Breeding. At Manu, 22 births of Weddell’s Saddle-back Tamarins in eleven groups occurred in the late dry season and rainy season from late August to March. Their breeding strategy was either monogamous (just one breeding pair) or polyandrous (more than one male mates with the breeding female and cares for the young). Usually, as is typical of callitrichids, there is only one breeding female in a group, but breeding by secondary females does occur. When this happens, secondary females give birth at least three months before or three months after the primary breeding female (always the older of the two), avoiding competition for infant carriers. There is evidently some competition between adult females for breeding positions. In eight of ten years of data from Manu, the number of mature females that did not breed exceeded the number of breeding females. There are some interesting differences between the sexes in terms of their reproductive strategies. Females that manage to obtain breeding positions usually do so when they are 2-5 years old, typically either in their natal group (taking over from their mother) or in a neighboring group. Territorial encounters between groups enable adult females to assess their chances of establishing themselves in neighboring groups. In some cases, however, females left their group and established a new one with emigrating males. Many females disappear when they are 2-5-4-5 years old because they leave the group to look for breeding positions and many die as a result. Joining neighboring groups is the main dispersal strategy of the females. From observations of groups in eleven territories during 13 years of study, only two females entered groups that were not their neighbors, and only one female entered a group that was not a former member of any of the groups that were studied. Average tenure of the breeding female in a group was 3-3-5 years. Groups contained more than one adult male far more often than two adult females. Copulations were recorded in seven groups, and in six of them, two males mated with the same female (i.e. polyandrous). The polyandrous males were sometimes related to each other, sometimes not. The high frequency of polyandry indicated that males may be more successful than females in leaving their groups to search for breeding opportunities. While only one female immigrated into the study population over the 13-year study, at least nine males did so. The need for help in the care of infants seems to favor this polyandrous system. Offspring of the breeding pair help in carrying their younger brothers and sisters, and when there are no older offspring in the group to provide this help, both males and females benefit from the aid of a second male. Another sex difference noted was in terms of secondary emigration. No females transferred from one group to another more than once, but males did, although infrequently (only four known cases during the 13-year study). This may be due to males forming polyandrous groups when there are no offspring to help with infant rearing, but when the group grows and the surplus male’s help is no longer beneficial, the dominant male may expell the other male. In each case when a male transferred groups for a second time,it had belonged to a one female—two male group that had bred successfully, and the male subsequently moved into another smaller group.
Activity patterns. Groups of the nominate subspecies weddelli spend c.20% of the day traveling, c.44% resting, and 32% feeding and foraging. More timeis spent resting and less time traveling when the dominant female is pregnant or when young infants were being carried by the group members. At the beginning of the day, they tend to feed on ripe fruits first and then switch to animal prey. Resting sessions occur throughout the day but are predominant at 11:00-13:00 h. They feed again at a fruiting tree in the late afternoon before retiring to their sleeping tree, normally at 16:00-17:00 h.
Movements, Home range and Social organization. Weddell’s Saddle-back Tamarins live in stable extended families, and many mature offspring tend to remain in their natal group. In Bolivia, group size is 2-9 individuals (average 4-5), and home ranges are 30-33 ha. In south-eastern Peru, 28 groups had 2-15 individuals, with an average of six. During their daily activities, Weddell’s Saddle-back Tamarins (subspecies weddell) travel up to 1300 m. Surveys in the Pando region in far north-western Bolivia found it to be the most common primate species, with 33 groups counted being as large as eleven individuals and averaging seven individuals. Groups use the center of their home range more than the periphery. At Manu, groups of the subspecies weddell: form a close association with the Bearded Emperor Tamarin, occupying overlapping territories that they defend jointly against neighboring groups. Combined group sizes ranged from five (three Bearded Emperor Tamarins and two weddelli ) to 16 (eight of each). These mixed groups are long-lasting; one was observed for more than three years. Both species participate in coordinating their association with the other. They do not usually intermingle but travel separately along parallel paths ( weddelli lower in the forest and more spread out and the Emperor Tamarins above them), and they rest and forage separately. Often they are out of sight of each other but maintain contact by their long-calls. When individuals of the two groups meet in a fruiting tree, for example, Emperor Tamarins are dominant over the smaller weddelli . Mixed groups observed at Manu National Park and Biosphere Reserve occupied stable home ranges of 30 ha to more than 100 ha, which they defended against neighboring groups. The ecology and behavior of the mixed groups of Weddell’s Saddle-back Tamarins (subspecies weddellr) and Red-bellied Tamarin (subspecies labiatus ) in Bolivia are very similar. Associations with groups of Rondon’s Marmosets are not as strong as they are with the species of mustached tamarins. The tamarins occupied a home range of c.44 ha and were with the marmosets for some time every day. Both species exploited the gum of the seed pods of Parkia pendula ( Fabaceae ), and the tamarins eat gum from the holes gouged by the marmosets. In south-eastern Peru, densities in ten localities ranged from 0-9 groups/km?* (5-1 ind/km?) to 5 groups/km?* (30-3 ind/km?*). At Manu, the bicolored hawk (Accipiter bicolor ) preys on young Weddell’s Saddle-back Tamarins. Density estimates of weddelli have been obtained for a number of localities. A density of c.16 ind/km? was estimated for Manu, while in south-eastern Peru between the rios Acre and Tahuamanu, densities were 0-76-3-6 groups/km? or 4-6-22 ind/km?. Subsequent surveys in that region provided higher densities of 2:2-9-4 groups/km?® or 13-5-56-2 ind/km?, averaging 3-8 groups or 22-6 ind/km?. Densities of 16-9-20-8 ind/ km? and 5-5 groups/km?, or 33 ind/km?, were estimated for the Pando region, Bolivia. The only estimate obtained to date in Brazil was at Sao Domingos on the upper Rio Jurua in Acre State where mean group size was 6-4 and the density was 43 ind/km? or 6-7 groups/km?®. Densities of 40-50 ind/km? or 6-9 groups/km? are probably close to the upper limits for Weddell’s Saddle-back Tamarins anywhere.
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List (as S. fuscicollis weddellz). In 1986, A. D. Brown and D. I. Rumiz reported that Weddell’s Saddle-back Tamarin was common in Bolivia. It is the most common of the callitrichids in a large area of the Pando region between the rios Acre and Madre de Dios. The following protected areas are within its geographical distribution: Madidi National Park, Pilon Lajas National Park, and Manuripi National Reserve in Bolivia; Abutfari Biological Reserve, Rio Acre Ecological Station, Cunia State Environmental Protection Area, Samuel State Ecological Station, Serra dos Trés Irmaos State Park, Guajara-Mirim State Park, and Pimenta Bueno Municipal Park in Brazil; and Manu National Park and Biosphere Reserve, Bahuaja-Sonene National Park, and Tambopata National Reserve in Peru.
Bibliography. Aquino & Encarnacién (1994b), Bicca-Marques (1999, 2000, 2005), Bicca-Marques & Garber (2003, 2004, 2005), Bicca-Marques et al. (1997), Buchanan-Smith (1990, 1991b, 1999), Buchanan-Smith & Hardie (1997), Buchanan-Smith et al. (2000), Calegaro-Marques et al. (1995), Castro et al. (1990), Coimbra-Filho (1990), Crandlemire-Sacco (1988), Encarnacion & Castro (1990), Ferrari & Martins (1992), Ferrari, Iwanaga & da Silva (1996), Ferrari, Lopes et al. (1995), Ferrari, Sena et al. (2010), Goldizen (1987a, 1987b, 1988, 1989), Goldizen & Terborgh (1986, 1989), Goldizen, Mendelson et al. (1996), Goldizen, Terborgh et al. (1988), Hardie (1995, 1998), Hardie & Buchanan-Smith (1997, 2000), Heltne et al. (1976), Hershkovitz (1966, 1977), Heymann & Buchanan-Smith (2000), Izawa & Bejarano (1981), Izawa & Yoneda (1981), Janson & Terborgh (19853, 1985b), Janson et al. (1981, 1985), Lopes & Ferrari (1994), Matauschek et al. (2011), Mena et al. (2007), Peres (1991a, 1993d), Peres et al. (1996), Pook & Pook (1982), Rehg (2006a, 2006b, 2009, 2010), Snowdon & Soini (1988), Tagliaro et al. (2005), Terborgh (1983), Terborgh & Goldizen (1985), Terborgh & Stern (1987), Valverde et al. (1990), Wallace et al. (2010), Yoneda (1981, 1984a, 1984b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.