Cherax latimanus, Raadik, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5026.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:9B0B019A-0172-4963-8CF2-2209C226FCE9 |
|
persistent identifier |
https://treatment.plazi.org/id/F9937BB4-4066-4330-9745-C184272E29FB |
|
taxon LSID |
lsid:zoobank.org:act:F9937BB4-4066-4330-9745-C184272E29FB |
|
treatment provided by |
Plazi |
|
scientific name |
Cherax latimanus |
| status |
sp. nov. |
Cherax latimanus sp. nov.
urn:lsid:zoobank.org:act:F9937BB4-4066-4330-9745-C184272E29FB
Swamp Yabby
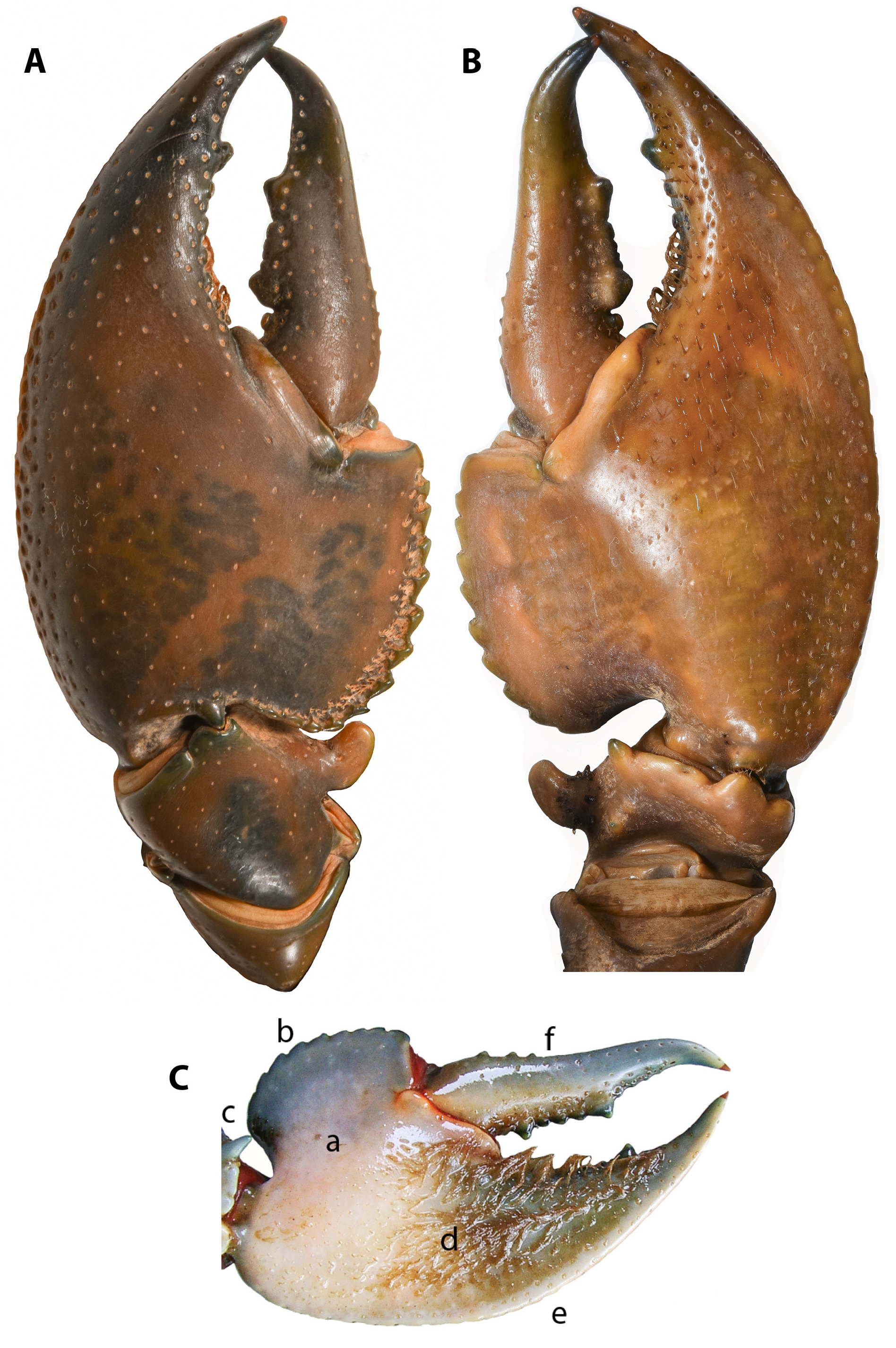
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , Tab. 1–5)
Cherax View in CoL B sp. nov. — Lawrence et al. 2006: 31.
Cherax destructor View in CoL — Mussared, 1997: 60; MDBA, 2009. (Non Cherax destructor Clark, 1936 View in CoL )
Cherax diogenes — Crabb, 1997: 187; Littlejohn, 2001: 754. (Unavailable)
Cherax View in CoL n. sp. — Littlejohn, 2001: 755.
Cherax rotundus View in CoL — Sokol, 1986: 227 (partim); 1988: 72 (partim); Austin et al., 2003: 102, 107; Munasinghe et al. 2004b: 556; Nguyen & Austin, 2005: 210; McCormack, 2005; 7; McCormack, 2008: 27; Schultz et al., 2009: 585; Jones, 2011: 289; Bentley, 2014: 107; Anon, 2017: 1. (Non C. rotundus Clark, 1941 View in CoL )
Cherax rotundus rotundus View in CoL — Austin et al., 2003: 102. (Non C. rotundus Clark, 1941 View in CoL )
Cherax sp. — Lawrence et al. 1998: 100; Edney et al., 2002: 200.
Cherax sp. C . — Horwitz & Austin, 1995: 32; Hale & Butcher, 2011: 20; Anon, 2018: 10.
Cherax View in CoL sp. nov. — Ayres et al., 2012.
Cherax sp. Swamp — Unmack et al., 2019: 859.
C. sp. [Swamp] — Unmack et al., 2019: 862.
C. sp. Swamp — Unmack et al., 2019: 863.
MDB C. sp. Swamp — Unmack et al. 2019; tab. 1.
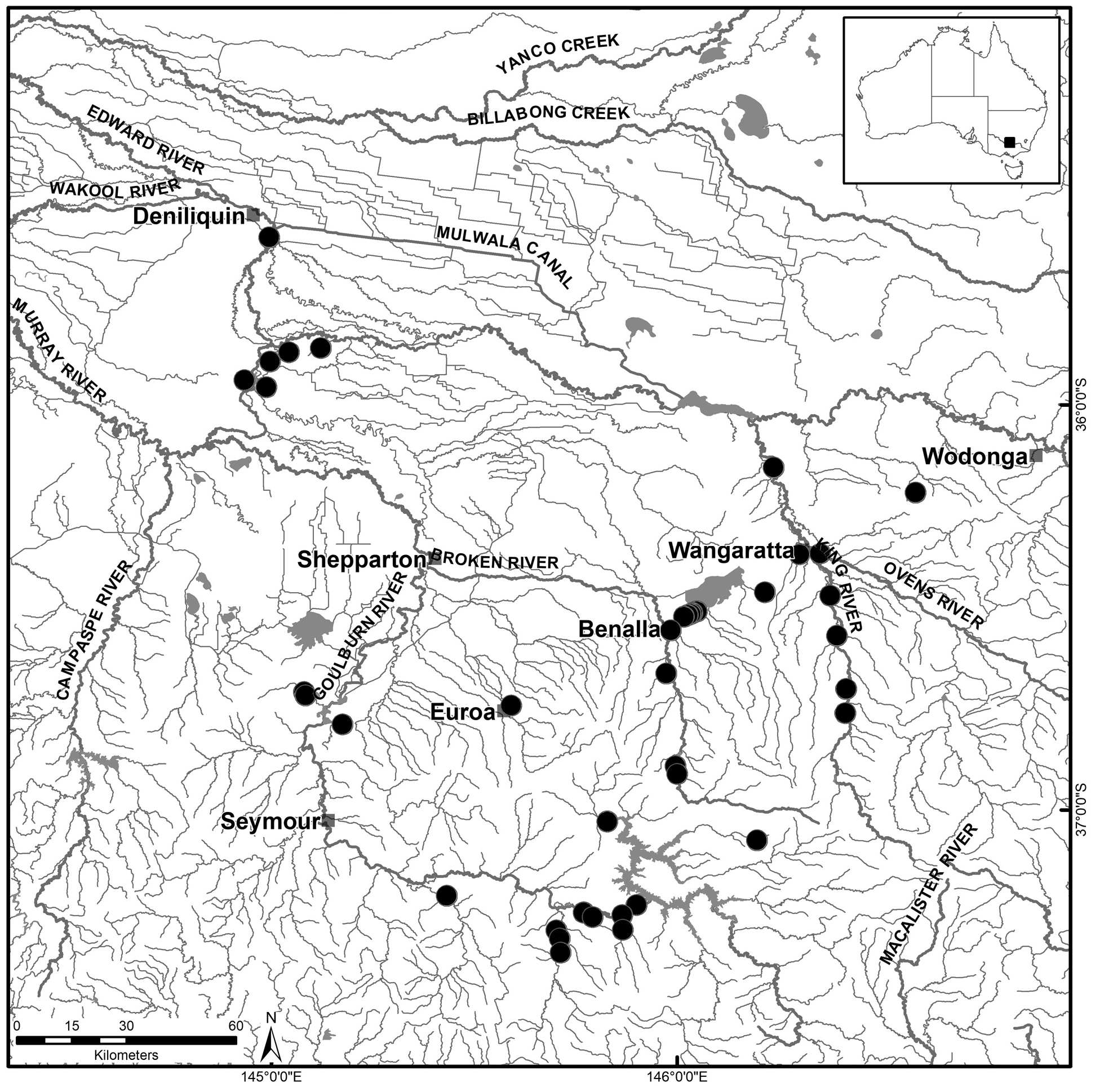
Type material. HOLOTYPE. NVM J74790 View Materials , male (63.6 mm OCL, 132 g), roadside drain on south-east side of Nelson Road, near Benalla / Yarrawonga Road , (near Benalla), Victoria (Broken R system), 36 o 31.001’S, 146°2.005’E, 175 m elevation, bait trap, RBM, 16 October 2009, (ex ACP 102468). GoogleMaps
PARATYPES. (All Victoria) AM P.104571, 1 male (53.7 mm OCL), collected with holotype (ex ACP 102467) GoogleMaps ; AM P.104572, 1 male (38.7 mm OCL) and NMV J74791 View Materials , 1 male (43.7 mm OCL), roadside drain, Nelson Road, near Lake Mokoan inlet channel, Victoria ( Broken R system), 36 o 30.613’S, 146°2.926’E, 175 m elevation, bait trap GoogleMaps , RBM, 12 October 2009 (ex ACP 102453 and ex ACP 102452 respectively) ; NMV J74792 View Materials , 7 males (55.5, 58.6, 63.5, 66.0, 66.1, 68.0, 70.5 mm OCL), 4 females (43.3, 48.7, 58.0, 59.7 mm OCL), Gulf Creek, Mill Log Track, Barmah Forest ( Broken R system), 35°51.58’S, 145°7.285’E, 110 m elevation, fine mesh, double wing fyke net, Matthew Jones & Andrew Pickworth, September to October 2003 GoogleMaps ; NMV J74793 View Materials , 2 females (43.6, 51.7 mm OCL), Lake Nillahcootie, Barjarg ( Broken R system), 36°54.65’S, 145°59.993’E, roadside drain, 275 m elevation, by hand GoogleMaps , RBM & SC, 10 August 2009 (ex ACP 102273–4) ; NMV J74794 View Materials , 1 male (52.5 mm OCL), next to Midland Highway, Lake Nillahcootie ( Broken R system), 36°53.518’S, 145°59.75’E, roadside drain, 280 m elevation, by hand GoogleMaps , RBM & SC (ex ACP 102270); NMV J74795 View Materials , 1 male (36.6 mm OCL), Maroondah Highway, Bonnie Doon ( Goulburn R system), 37°1.725’S, 145°49.66’E, drain beside road, 305 m elevation, by hand GoogleMaps , RBM & SC, 10 August 2009 (ex ACP 102276) ; NMV J74796 View Materials , 1 female (43.9 mm OCL), Reedy Lake Wildlife Reserve ( Goulburn R system), 36°42.557’S, 145°04.83’E, 145 m elevation, by hand GoogleMaps , RBM & SC, 9 August 2009 (ex ACP 102253) ; NMV J74797 View Materials , 2 males (50.6, 59.8 mm OCL), boundary of Reedy Lake Wildlife Reserve ( Goulburn R system), 36°43.033’S, 145°05.058’E, roadside drain, 145 m elevation, by hand GoogleMaps , RBM & SC, 9 August 2009 (ex ACP 102252, 102254) ; NMV J74798 View Materials , 1 male (50.3 mm OCL), Rubicon River floodplain, Christies Road, Thornton ( Goulburn R system), 37°15.174’S, 145°46.174’E, by hand, 210 m elevation GoogleMaps , RBM & SC, 9 August 2009 (ex ACP 102257) ; NMV J74799 View Materials , 1 male (50.3 mm OCL), Rubicon River floodplain, Christies Road, Thornton ( Goulburn R system), 37°15.118’S, 145°46.185’E, by hand, 200 m elevation GoogleMaps , RBM & SC, 9 August 2009 (ex ACP 102258) .
Other material examined. New South Waes: ACP 100343, male (66.6 mm OCL), Lake Moira, near Mathoura , 35°56.34’S, 144°56.022’E, 95 m elevation, 7 December 2005 GoogleMaps ; ACP 100438, 1 male (66.8 mm OCL), Lake Moira, near Mathura , 35°56.34‘S, 144°56.022’E, 95 m elevation, 6 March 2006 GoogleMaps . Victoria: NMV J74800 View Materials , 5 females (11.3–15.9 mm OCL), 1 male (11.9 mm OCL), collected with holotype (ex ACP 102461–102466) GoogleMaps ; NMV J75300 View Materials , 5 females (15.8, 11.7, 15.5, 14.4, & 12.7 mm OCL), collected with holotype (ex ACP 102461, 102463–102466) GoogleMaps ; NMV J5301 About NMV , 1 male (11.9 mm OCL), same as (ex ACP 102462) ; NMV J74801 View Materials , 4 males (55.0– 72.9 mm OCL), 1 female (45.9 mm OCL), Gulf Creek, Mill Log Track, Barmah Forest ( Broken R system), 35°51.58’S, 145°7.285’E, 110 m elevation, fine mesh, double wing fyke net, Matthew Jones & Andrew Pickworth, September to October 2003 GoogleMaps ; NMV J23754 View Materials , 1 male (54.3 mm OCL), Budgee and Snag creeks, Barmah State Forest ( Broken R system), ~ 35°53.602’S, 144°59.81’E, single wing fyke net, 105 m elevation, L. McKinnon, 21 October 1991 GoogleMaps ; ACP 102452–102453, 2 males (43.5, 38.1 mm OCL), Nelsons Rd, Lake Mokoan , 35°30.6132 ; S, 146°02.9256’E, roadside drain, 175 m elevation, by hand, RBM, 12 October 2009 ; NMV J74802 View Materials , 3 females (13.8–15.4 mm OCL), Nelsons Road, Lake Mokoan ( Broken R system), 36°30.89’S, 146°02.264’E, roadside drain, 175 m elevation, by hand, RBM, 15 October 2009 (ex ACP 102455–102457) GoogleMaps ; ACP 102857, male (21.5 mm OCL), Murray Road, Benalla , 36°31.1778’S, 146°01.5798’E, roadside drain, 175 m elevation, by hand, RBM, 9 May 2010 GoogleMaps ; NMV J74803 View Materials , 2 males (23.4, 39.7 mm OCL), Reedy Lake Wildlife Reserve ( Goulburn R system), 36°42.557’S, 145°04.83’E, 145 m elevation, by hand, RBM & SC, 9 August 2009 (ex ACP 102251, 102283) GoogleMaps ; ACP 102758, male (70.1 mm OCL), Wangaratta / Whitfield Road, Whitfield , 36°45.6912’S, 146°24.8736’E, roadside drain, 255 m elevation, by hand, RBM, 13 March 2010 GoogleMaps ; ACP 102759, male (34.2 mm OCL), Wangaratta / Whitfield Road , 36°42.0774’S, 146°25.0044’E, roadside drain, 230 m elevation, by hand, RBM, 13 March 2010 GoogleMaps ; ACP 102275, 1 male (45.3 mm OCL), roadside drain Lake Nillahcootie, Barjarg , 36°54.6498’S, 145°59.9928E, 275 m elevation, RBM & SC, 10 August 2009 GoogleMaps ; ACP 102863–102864, male (41.3 mm OCL), female (38.3 mm OCL), near a tributary of Ford Creek, Mount Buller Tourist Road, east of Mansfield , 37°04.4712’S, 146°11.7768’E, roadside drain, 430 m elevation, by hand, RBM, 10 May 2010 GoogleMaps ; ACP 102880, female (28.6 mm OCL), creek off Park Avenue, Eildon ( Goulburn R system), 37°14.0454’S, 145°53.9988’E, 240 m elevation, by hand, N. Taylor & RBM, 11 May 2010 GoogleMaps ; ACP 102867, male (28.7 mm OCL), unnamed stream, Goulburn Valley Highway, west of Snobs Creek Hatchery , 37°15.5256’S, 145°51.882’E, 215 m elevation, by hand, RBM, 10 May 2010 GoogleMaps ; ACP 102870, female (34.7 mm OCL), water point dam, off unnamed track off Herbs Road, Rubicon State Forest, south of Snobs Creek , 37°17.7126’S, 145°51.969’E, 800 m elevation, by hand, Nicholas Taylor & RBM, 10 May 2010 GoogleMaps ; NMV J74804 View Materials , 1 About NMV cephalothorax only (55.1 mm OCL), cattle paddock on Rubicon River floodplain, Christies Road, Thornton ( Goulburn R system), 37°15.164’S, 145°46.181’E, 210 m elevation, by hand, RBM & SC, 9 August 2009 (ex ACP 102259) GoogleMaps ; NMV J13455 View Materials , 1 female (37.6 mm OCL), Acheron River tributary, crossing Maroondah Highway, 7.9 km north of Buxton Post Office ( Goulburn R system), ~ 37°21.078’S, 145°42.738’E, 240 m elevation, by hand, P. Horwitz GoogleMaps ; NMV J10666 View Materials , 1 male (57.0 mm OCL), crossing on highway 2 miles north of Taggerty ( Goulburn R system), ~ 37°17.783’S, 145°42.144’E, 205 m elevation, A.A. Martin & P. Rawlinson, 29 September 1963 GoogleMaps ; ACP 102744, male, (45.4 mm OCL), Maroondah Highway, Taggerty , 37°18.9906’S, 145°42.6204’E, roadside drain, 210 m elevation, by hand, RBM, 12 March 2010 GoogleMaps .
Comparative material: see Appendix 1
Diagnosis. Rostrum very short and broad, gently curved downwards anteriorly, apex lacking spine; rostral carinae raised and smooth. Post-orbital ridges conspicuous, low, unarmed, very widely spaced posteriorly, extending between low, but raised, bosses. Suborbital spine very small. Antennae long, reaching to telson or last abdominal somite. Cervical spines tiny or absent. Areola extremely narrow, usually 0.06 × areola length and 0.05 x carapace width, narrowest near 0.75 distance anteriorly. Pore present on each lateral process of pereopods 3 and 4.
Chelipeds very broad, 55–59% of length. Dactylus long, uniquely with 4 (0–8) marginal mesial dactylar basal spines, mesial margin straight or slightly concave, narrowing distally and turning abruptly to a sharp, terminal spine and curving dorsoventrally from the horizontal plane; cutting edge with 2 prominent molariform teeth. Propodus very wide, usually> 0.5 × length, about 2 × as wide as deep; propodal palm often distinctly extending posteriorly well past level with the propodal/carpal articulation; posteromesial propodal margin with 15 (12–20) raised blunt spines; lateral propodal margin fully calcified, lunate, with punctations with raised rims forming a characteristic rough surface. Propodus smooth ventrally, concave on the posterior to mid portion of palm from a broad, raised ridge extending obliquely from the base of the fixed finger to the carpal articulation.. Dorsal surface of propodus with a thin band of tufted setae along inside edge of propodal mesial margin; setation on ventral surface distinctive, with long, dense setae present along length of ventral cutting edge, extending over propodal finger, and palm on the lateral side of the oblique ridge to near the carpal articulation; sparse to moderately dense towards base. Carpus non-setose, with single, large, paddle-shaped mesial carpal spine and 3 (1–6), blunt and small to moderate sized ventromesial carpal spines; merus similarly lacking setation.
Description. OCL/TL 0.42 (0.39–0.57).
Carapace: Subovate, slightly laterally compressed, wider than deep, about half of body length. CaD/CaW 0.92 (0.84–1.02); CaD/CaL 0.49 (0.43–0.57); CaW/CaL 0.53 (0.45–0.63).
Rostrum. Broad, very short, reaching to near or to base of penultimate antennular peduncle article, gently curved downward, lateral margins convergent. Apex blunt, without discernible spine, terminating in smooth round- ed, downward edge. Rostral carinae raised, terminating abruptly before rostral apex and fusing with rostral rim, posteriorly declining in height, usually very low opposite eyes and further back; carinae smooth, divergent at base, often longer than rostrum, terminating short of, or just reaching anterior end of postorbital ridge. Intracarinate surface broad, smooth, slightly convex, lightly punctate, non-setose. Ventral edge of rostrum setose from apex posteriorly to behind orbit, with ventrally oriented, closely spaced setae forming ‘curtain’, longer in front of eye towards apex, shorter near apex and posteriorly opposite ocular peduncle. OCL/CaL 0.89 (0.86–0.92); RosW/RosL 0.75 (0.64–0.93); RosL/OCL 0.16 (0.13–0.19).
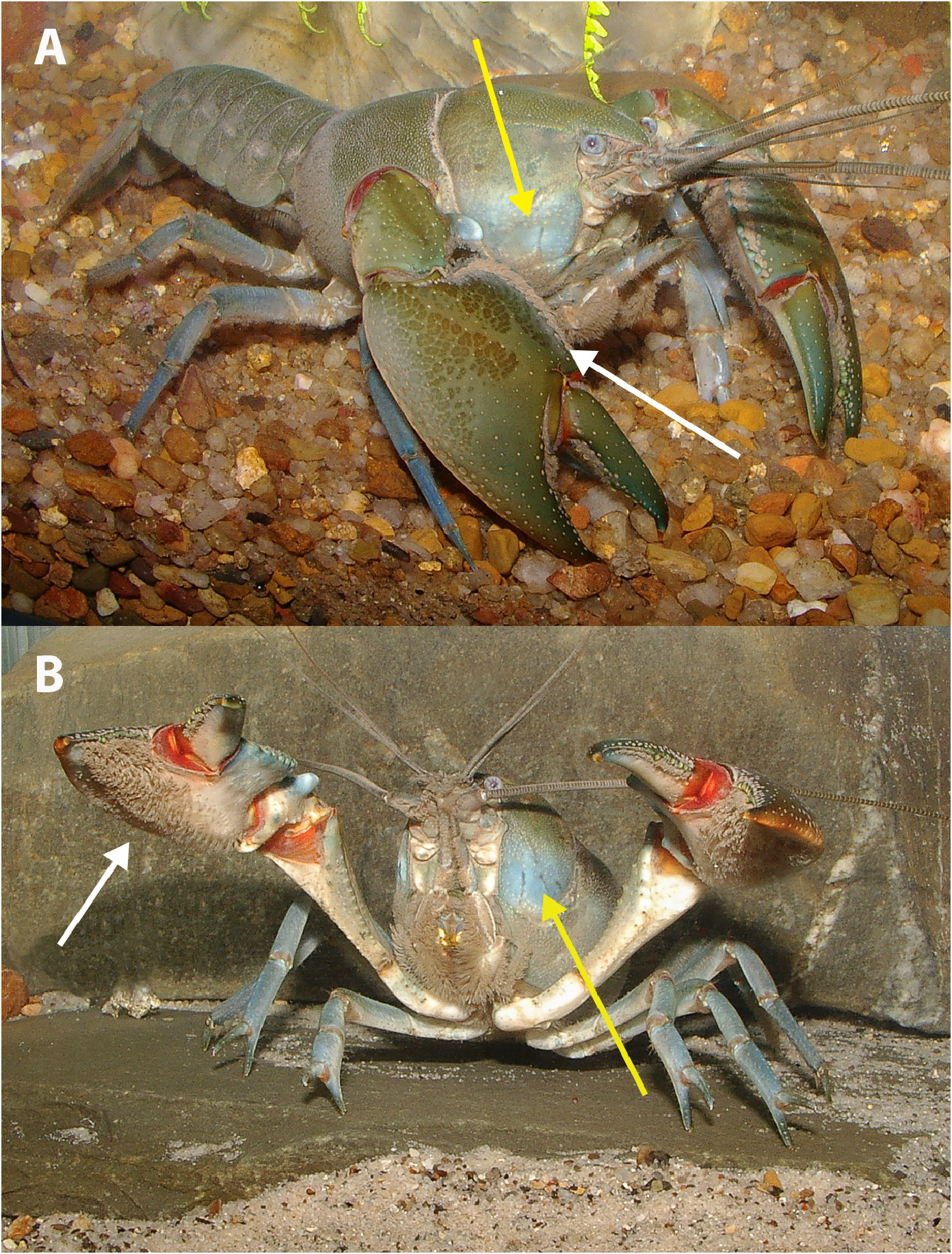
Cephalon. Smooth, finely punctate, with very small tubercles laterally on mandibular and antennal regions. Live animals with distinctive blueish tinted area on antennal, mandibular, and portions of hepatic and branchiostegal surfaces ( Figs. 1 View FIGURE 1 , 6 a,b View FIGURE 6 ). Postorbital ridges conspicuous, divergent, slightly raised, continuous, usually punctate along lateral edge, originating from low, rounded boss with or without blunt small edge, extending posteriorly and terminating at larger, higher, slightly ovoid boss. Usually with two small raised smooth submedian swellings on either side of the mid-line, anterior to postcervical groove. Suborbital spine very small, sharply, or often bluntly, pointed. PORL/OCL 0.23 (0.18–0.28); PORW/PORL 1.35 (1.14–1.73).
Antennae. Antennal flagella very long, approximately 1.8–2.0 OCL, when folded along body reaching abdominal somite 6 or telson, but often damaged distally, appearing shorter.
Scaphocerite extending to midlength of penultimate antennular peduncle article, widest distal to midlength, lateral margin thick, slightly convex and terminating in short sharp conical spine. Epistome naked; interantennal spine short and broad, wider than long, round to angular, with raised, smooth, scalloped margins, apical spine small to medium, tapering and roundly pointed. Coxopodite spines absent but edge can be serrated, usually with 1–3 very small, unequal and blunt protuberances; basiopodite antennal spine absent. Third maxilliped ischium setose, rounded at laterodistal corner, exopodite 1.3 times as long as ischium.AntScL/OCL 0.12 (0.10–0.14), AntScL/RosL (0.77 (0.65–0.94).
Thorax. Densely punctuated along dorsal and upper branchial surfaces, with setose tubercles along mid to lower sides, sprouting multiple, bristly setae. Cervical groove deeply impressed and v-shaped at midline; cervical spines absent, cervical tubercles usually present, often small, larger in bigger animals, approaching small spines, particularly on animals> 65 mm OCL (> 150 g). Tubercles often extending ventrally and along anteroventral margin of branchiostegite, with smaller, general tubercles with setae spread over anteroventral branchiostegal area. Branchiocardic grooves gently impressed; areola extremely narrow, ArL, narrowest at approximately 0.75 distance from posterior carapace edge towards cervical groove. AreL/CaL 0.41 (0.35–0.44); AreW/AreL 0.06 (0.03–0.09); AreW/CaW 0.05 (0.02–0.07).
Sternal keel. Prominent, deeply excavated between LP1 and LP2, raised immediately following LP1 to a narrow, posteroventrally oriented process, and posterior to LP2 as a low raised bump, followed by a deep notch at the anterior edge of LP2. Keel between LP2 and LP3 moderately excavated, sharp, raised anteriorly to narrow, elongate, setose rise, much higher (when specimen inverted) than all others on sternal keel, and raised posteriorly as a small bump, immediately followed by deep notch at LP3. Keel slightly raised between LP3 and LP3, profile angular, with convex margin sloping to and ending at LP4. Lateral processes of pereopods setose: LP1 small, with pointed, setose tips; LP2 larger, raised into short, setose ridge; LP3 slightly bulbous, raised into longer, setose ridge; LP4 large, flat, oriented posteroventrally, each angled toward midline; LP5 fused medially, separate from sternal keel, smaller than LP4, oriented mainly posteriorly, each angled toward midline, without triangular base plate. Pore present on lateral surface of LP3 and posterior surface of LP4, usually closed, visible as thin, short groove.
Abdomen. Females broader than males, in both, noticeably narrower than carapace, approximately 0.8 cephalothorax width. Dorsal surface of somites smooth, with very light, fine, shallow punctation, pleura with light setation. Anterior extension of pleuron of somite 2 partially overlapping posterior edge of pleuron 1. AbdL/TL 0.37 (0.17–0.39); AbdL/CaL 0.79 (0.72–0.87); AbdW/OCL in males 0.48 (0.46–0.50), females 0.51 (0.49–0.53).
Tailfan. Telson U-shaped, longer than wide, with small, sharp, broad lateral marginal spine at approximately posterior one-third on each lateral margin. Telson punctate dorsally over anterior two-thirds, posterior one-third heavily setose. Exopod longer than telson, endopod slightly longer or as long as telson. Endopod with large, broad, sharp spine at caudolateral corner, longitudinal median carina terminating in large, sharp, upturned central spine. Exopod with medium spine at caudolateral corner, with longitudinal median carina terminating in medium sharp spine slightly upturned on suture; suture relatively straight with 6–14 similar sized outer and inner spines, inner spines reducing in size and terminating halfway across uropod.
Fringe of setae extending along entire margin of telson, along entire outer margin of exopod to about the inner caudolateral corner, and from outer caudolateral corner of endopod to beyond inner caudolateral corner; setae on outer margins of telson and exopod extending onto ventral surface anterior to caudolateral corner. Small, dense patch of setae on dorsal surface of the anteromesial portion of uropod, with corresponding, but smaller patch on ventral surface of telson where it overlaps. Dorsal surface of tailfan generally moderately setose; sparse, long setation over ventral surface. Uropodal protopod spine usually tiny, or absent. TelL/TL 0.16 (0.14–0.20); TelW/TelL 0.80 (0.61–0.86).
Chelipeds. Subequal in length; generally equal in form and size (except when regenerating); distinctively very broad in shape, approximately 55–59% of length. Fingers longer than palm and distinctly gaping.
Dactylus. Moderately punctate, longer than propodal palm length; broad at base, width about constant to opposite most posterior or anterior prominent tooth, then narrowing as mesial margin tapers rapidly inward, and dactylus turning abruptly outward to form distinct, sharply pointed narrow hook with terminal spine; dactylus curving anteroventrally in horizontal plane along its length with tip resting just below that of the propodal finger when closed. Mesial margin straight to slightly concave. Usually 4 (0–8) marginal mesial dactylar basal spines, extending from base to about midlength; these spines rarely absent on both chelae (<15% of individuals examined). Cutting edge usually with 2 (1–3) prominent, distinctive teeth, offset slightly ventrally from 8 (4–11) small teeth. Setae present along dorsal cutting edge, very sparse and restricted to near base; present along length of ventral cutting edge, sparse to moderately dense towards base. DacL(L)/DactL(R) 1.02 (0.78–1.33); DacL/OCL: (L) 0.50 (0.35–0.63), (R) 0.50 (0.33–0.62); DacL/ProL: (L) 0.612 (0.53–0.66), (R) 0.62 (0.56–0.69); DacL/ProPL: (L) 1.56 (1.14–1.78), (R) 1.60 (1.23–2.11).
Propodus. Broad, width usually>0.5 length; strongly dorso-ventrally compressed, greatest depth on midline near midlength of palm; palm shorter than propodal finger. Length of propodal palm shorter than fixed finger, extending beyond mesial margin of dactylar base; posterior margin extending well past level of propodal/carpal articulation. Mesial and posterior propodal margin with upturned edge formed by 15 (12–20) rounded spines; smallest on posterior edge near articulation, extending to, or near, full length of mesial margin; usually with a narrow band of sparse setae, occasionally dense or absent ( Fig. 6a View FIGURE 6 ). Inner edge of spine row with series of deep pits, occasionally bounded by an inner, second, partial series of smaller spines and pits. Lateral propodal margin fully calcified and distinctly lunate, evenly curved to tip of fixed finger; lateral and dorsolateral margin with large punctuations extending to near tip of finger, with posterior edge of lateral margin punctate very slightly raised as tiny, flat tubercles, particularly along palm edge, forming a rough surface; difficult to count. Propodal finger broad at dactylar articulation, curving and narrowing to a pointed tip with a terminal spine, distinctly curving mesially; propodus only slightly curved ventrally in the horizontal plane, near the tip of the finger. Dorsal surface of propodus evenly arched, and smooth, ventrally smooth, concave on the posterior to mid portion of palm from broad, raised ridge extending slightly obliquely from about base of fixed finger to carpal articulation; propodal palm laterally from ridge evenly sloping to lateral propodal margin. Cutting edge of propodal finger with 1 (0–1) prominent, distinctive tooth, offset slightly ventrally from 10 (5–13) small teeth. Setae present along dorsal cutting edge, very sparse, restricted to base; narrow, sparse band of setal tufts along inside edge of propodal mesial margin to posterior edge. Long, dense setation along length of ventral cutting edge, extending over propodal finger, and palm on lateral side of oblique ridge to near carpal articulation ( Fig. 6b View FIGURE 6 ); sparse to moderately dense towards base. ProL(L)/ProL(R): 1.04 (0.86–1.37); ProL/OCL: (L) 0.83 (0.55–1.04), (R) 0.80 (0.51–1.05); ProW/ProL: (L) 0.57 (0.46–0.62), (R) 0.58 (0.47–0.64); ProD/ProL: (L) 0.29 (0.24–0.38), (R) 0.29 (0.17–0.41); ProD/ProW: (L) 0.50 (0.32–0.70), (R) 0.49 (0.33–0.69); ProPL/ProL: (L) 0.39 (0.35–0.46), (R) 0.39 (0.31–0.46).
Carpus. Dorsal groove very lightly impressed. Single mesial carpal spine large, paddle shaped, with broad, rounded apex usually curving anteriorly. Single, large and bluntly point ventral carpal spine present, articulation spine tiny and blunt to sharp, or absent. Usually 3 (1–6) small to medium, blunt ventromesial carpal spines, often with 1–2 between mesial carpal and ventral spines and from 1–4 small spines in rough row posteriorly towards carpal/meral articulation. Dorsal carpal spines absent; lateral carpal spine usually small to tiny, or absent. Setation absent except for few small setae on anteroventral edge of carpus at carpal/propodal articulation. CarL/ProL: (L) 0.35 (0.31–0.39), (R) 0.36 (0.31–0.40).
Merus. Lacking setation. Dorsal surface with 6 (0–12) tiny to small, raised lumps to blunt spines extending posteriorly along meral dorsal margin, most distal spines usually larger and sharper. Outer meral spine usually absent, if present, tiny, and bluntly rounded, occasionally pointed. Small to medium sized, blunt to sharp spines spread across ventral surface.
Ischium. Ventral surface with 1–3 rows of rounded spines, basis unarmed.
Pereopods 2–5. Pereopods 2–3 chelate, all segments smooth, with very sparse bristle setae, longest on dactylus and propodus. Pereopods 4–5 simple, setation similar to pereopods 2–3 except dactylus and propodus densely covered by bristle setae. Basis of all pereopods with moderate covering of short, stiff setae; female gonopore fully or partially ringed with setae, male gonopore usually naked. Male coxa 5 with cuticle partition present between gonopore and arthrodial membrane of sternum.
Size. To 79 mm OCL and 272 g in weight, usually to 45–55 mm OCL; reported to 82 mm OCL and 310 g in captivity (Steve Chara, pers. com. 2012).
See Tables 1–3 for frequency distribution of selected meristic characters, and summary meristic variation in type and non-type material, and Table 4 & 5 for comparative value ranges of morphometric characters. See Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 and 6 View FIGURE 6 for external morphology.
Colour in life. Adult body colour variable between populations; generally, darker dorsally, dark green, dark blue or dark brown, becoming lighter laterally, generally with a light blue tint along sides of cephalon ( Fig. 6 View FIGURE 6 ). Body cream to tan ventrally, walking legs lighter, light blue, steel grey or green. Chelae generally green or brown, tending towards yellow, or blue, brown on propodal lateral margin with blue tint on propodal dorsal surface. Cutting teeth usually creamy-white, turquoise or khaki. Mesial propodal spines turquoise, light blue or blue-grey and always lighter than propodus; punctations also lighter. Ventral surface of chelae varying from pink to orange to cream. Joints of chelae, and between chela-carpus-merus on pereopod 1, bright red; joints on walking legs orange or cream. Distal end of dactylus and propodal finger often blue, apex cream to clear and orange tipped. Dactylar marginal basal spines light blue to green, sometimes cream. Mesial carpal spine usually lighter than dorsal surface of carpus; dorsal meral spines cream to light brown. Distinctive dappled pattern present across dorsal surface of propodal palm and carpus ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ); also, often present on posterior portion of dorsolateral margin of the cephalon, sometimes difficult to discern.
Juveniles light green, blue or brown dorsally. Small (<15 mm OCL; 1–2 g) animals with a bright red speckled colouration to chelae, legs and antenna, with a darker red to crimson speckling of the body ( Fig. 4 View FIGURE 4 ). Red speckling generally fades by 23 mm OCL (~ 5 g). Dactylar and propodal cutting edges often highlighted green to blue.
Etymology. From the Latin latus, meaning broad, wide, and manus, meaning hand, in reference to its distinctive wide chelae, an obvious characteristic which separates it from the Common Yabby ( Cherax destructor ) with which it is broadly sympatrically found. Common name—Swamp Yabby, however, colloquially also referred to as Banjo Claw Yabby, Barmah Cray, Barmah Swamp Yabby, Broad Claw Yabby, Broad Palmed Yabby, Moon Claw Yabby, Mud Burrow Yabby, Spanner Yabby, Spanner Claw Cray, Spanner Claw Yabby, Swamp Cray, Swamp Yabby, or Swampies.
Distribution and habitat. Currently known from an area of approximately 17,700 km 2 in the mid-Murray River catchment, bounded by Deniliquin–Echuca–Nagambie–Taggerty–Eildon–Merrijig–Chiltern–Whitfield–Deniliquin ( Fig. 5 View FIGURE 5 ). Populations have been found along the Murray River from Lake Moira upstream to near Wodonga in New South Wales and Victoria, including along the Edwards River and Gulpa Creek, Barmah Forest, and the Murray River tributary catchments (i.e. Ovens, Broken and Goulburn rivers). Possibly more widespread, potentially also inhabiting the lower reaches of the Kiewa, Campaspe and Loddon river catchments in Victoria.
The species is restricted to flood plain reaches of rivers and creeks (e.g., up to 20 km each side of the Murray River), the periphery of shallow lakes, permanent to temporary swamps and wetlands, including claypans and shallow roadside drains ( Figs. 7–8 View FIGURE 7 View FIGURE 8 ). It has been recorded from lowland to foothill reaches, at elevations from 100–420 m above sea level (asl); one population, possibly translocated, has thrived in a forestry dam at 800 m asl. for about 20 years, south of Snobs Creek.
Cherax latimanus is rarely found in streams, billabongs, or dams, except during large floods. Instead, it usually resides in deep burrow systems along the periphery of swamps, lakes, and streams, including other wet environments such as earthen roadside drains ( Fig. 7 View FIGURE 7 ), floodplains, and low-lying forest or cleared grazing paddocks. These areas are usually quite flat, composed of impervious clays, and intermittently inundated with several mm of water during rain events, holding water during wet conditions but drying out completely, with dry surface conditions prevailing for most of the year.
Cherax latimanus has also been reliably recorded from the following locations (based on interview with collectors (in parentheses), no extant specimens but some records with images):
New South Wales: floodplain, Edwards and Gulpa Creek junction, ca. 2000, four large specimens dug from burrows (~ - 35°35.234’S, 144°59.665’E) (Dale McNeil). Victoria: (Ovens River basin) floodplain, tributary of Black Dog Creek just off Pine Road 20/04/2010 (- 36°12.96’S, 146°35.25’E) (Martin O’Brien); King River floodplain, off Oxley/Meadow Creek Road, 2019, (- 36°28.312’S, 146°22.630’E) (Robbie Alexander); near Moyhu (~ - 36°34.228’S, 146°23.606’E) (Dale McNeil); near Glenrowan (~ - 36°27.808’S, 146°12.966’E ((Dale McNeil); floodplain of Ovens River, off McLaughlins Track, 15/10/2009 (- 36°9.364’S, 146°14.25’E) (Zeb Tonkin); Ovens River floodplain near Wangaratta (~ - 36°22.047’S, 146°.21.126’E) (Geoffrey Edney ); Wangaratta Commons, Wangaratta 08/2019 (- 36°22.167’S, 146°18.0912’E) (Robbie Alexander); (Broken River basin) Barmah Forest, in Budgee Creek, Snag Creek, Smith’s Creek and Little Budgee Creek, 1992–1995 ( McKinnon 1997); Barmah Forest, just off Sand Ridge Track 09/1995 (~ - 35°57.403’S, 144°59.2482’E) ( Lawrence et al. 1998); swampy area on west side of Govers Track, Barmah Forest, 2/05/2000 (- 35°52.206’S, 145°2.598’E) (Susan Lawler); swamp near Benalla, 30/03/1999 (~ - 36°33.4314’S, 145°59.0502’E) (Beverly Westland); swamp on west side of Midland Highway, Yin Barun, 1995 (~ - 36°39.774’S, 145°58.386’E) (Liz Raven); (Goulburn River basin) roadside drain on tributary of Faithfulls Creek, just north of Euroa and east of Hume Freeway (area now under freeway fuel station), 24/11/2005 (- 36°44.5542’S, 145°35.4804’E) (Richard Francis); close to roadside between Euroa and Nagambie, 21/01/2000, found when trenching, retrieved by Fisheries Officer from works crew who were boiling specimens for lunch (Steve Smith); Yea River, Yea River picnic area next to Yea, 21/12/2009 (- 37°12.685’S, 145°25.909’E) (Di Crowther); 1.6 km (1 mile) east of Nagambie, 25/05/1998, found while trenching, three caught, 175–305 g (~ - 36°47.320’S, 145°10.504’E) (George Tuckett); floodplain of Rubicon River, 1 km south of Thornton, 2/01/2005 (~ - 37°15.834’S, 145°47.500’E) (Phil Littlejohn).
Unsubstantiated reports also suggest the species may be found further westward at Heathcote in the Campaspe River basin, and possibly as far west as the Loddon River system ( Howe, 1995) and at Tang Tang Swamp Wildlife Reserve west of Runnymeade (~ - 36°21.897’S, 144°17.779’E) (Alf Howe).
General biology. The sex ratio of adult Cherax latimanus appears to be male biased, estimated at 2.3:1 based on data from professional fishermen during flood events in Barmah Forest (Steve Chara, pers. com. 2009), noted during a survey of fish movement out of the Barmah Forest as water levels receded -(Matthew Jones, pers. com. 2003), and 1.5:1 based on our own observations from captures from burrow excavations. However, the sex ratio of smaller individuals (young adults <18 mm OCL (<3 g) excavated from burrows was estimated to be female biased at 1:2.3.
Males reach sexual maturity around 26–28 mm OCL (~ 7–10 g) and females at 30 mm OCL (~ 15 g), with breeding commencing in mid to late spring (October). A 64.9 mm OCL (104 g) female was recorded carrying ~ 500 eggs: their colour varied from olive-green to dark purple/glossy black. General observations on eggs indicate they are oval in shape, attached by a filament, fertile eggs are turgid and dark, but clear at the filament end and 1.6–2.5 mm in length, and infertile eggs are light in colour (Phil Littlejohn, pers. com. 1999). Longevity is unknown.
Cherax latimanus is an extensive burrow builder in water-holding clay. A typical burrow system consists of three to eight surface entrances (up to 80–100 mm wide) leading down tunnels to a central chamber that is often 0.6–1.0 m below the surface and 0.3 to 0.6 m in diameter. The entrance tunnels can extend straight to the surface from the chamber, or extend laterally, parallel to the surface, before rising, and some burrows have two or more chambers, at various levels, with interconnecting passages. Often, a chamber has at least one tunnel extending deeper, leading to the water table. The maximum depth of burrow systems can vary greatly, with most in drier areas being> 1.5 m deep, and those near permanent water, or those of smaller animals under ~ 50 mm OCL (~ 70 g) are only about 1.0 m deep: an attempted excavation of burrows at Lake Moira, NSW, extended down 3.2 m. Surface entrances of tunnels are usually partly surrounded by a fan of excavated material, sometimes concentrated outward in specific directions.
In the relatively drier north of its range near Mathoura and Deniliquin in New South Wales, the burrows are frequently capped with soil at the surface to conserve moisture ( Fig. 8 View FIGURE 8 ). Burrow systems in wetter areas further south in Victoria, are mostly open at the surface, with excavated material surrounding the entrance as a small soil chimney ( Fig. 9 View FIGURE 9 ), though they can also be capped ( Fig. 10 View FIGURE 10 ). During dryer times these tunnels can also be blocked with a soil plug at a depth of usually 0.7–1.0 m, though tunnels near Euroa were surface capped (Richard Francis, pers. com. 2005). Interestingly, approximately 30% of capped burrow systems observed during summer in the northern Millewa Forest had small sticks embedded in the dried soil of the cap (Ivor Stuart, pers. com. 2019) ( Fig. 11 View FIGURE 11 ).
We have recorded only one adult per burrow, and large females typically occupy large burrow systems. Many hundreds of juveniles hatch in a burrow with the female parent, but after six months or one year there are only three to 10 juveniles (<26 mm OCL) left; what becomes of the rest of the juveniles is unknown, and cannibalism by the parent or juvenile conspecifics is possible. When inundation occurs, it is thought that remaining juveniles leave the burrow during day and night and will start their own burrow in new areas, close to or further away from their natal burrow; individuals by 26–28 mm OCL occupy their own burrow.
Females appear to have high burrow fidelity and rarely venture outside, whereas males will leave burrows at night during wetter periods, often wandering widely on the surface, particularly on the periphery of the main habitat areas where the majority of adult females occur. After rain events recently excavated burrows are found around older burrow systems and these are often new burrows of juveniles or mature males.
Whilst normally absent from standing water habitats, surface movement of adults in flooded streams and swamps occurs during wetter periods when the species becomes more active. This is when the C. latimanus is usually encountered, often caught by recreational and commercial fishers following flood events.
Cherax latimanus has been reported to be very aggressive, particular to C. destructor with which it is found in broad sympatry. Often, if both species are caught together in a trap, the latter is usually killed in the wild by the former.
Diet is unknown, but anecdotal information from captive specimens suggests that vegetation (i.e., roots) is preferred over meat (i.e., earth worms) (George Tuckett pers. com. 1998).
Crayfish associates. Cherax latimanus is found in broad sympatry with the Common Yabby, Cherax destructor , but the two occupy different micro-habitats and so are rarely caught together, except in creek channels during major floods (e.g., within the Barmah Forest). Cherax destructor is generally found in permanent to intermittent creeks, dams, wetlands and rivers, whilst C. latimanus occupies off-channel, low-lying, more terrestrial and ephemeral, areas. It is also sympatric with the Upland Burrowing Crayfish, Engaeus lyelli, Clark, 1936 , and the Central Highlands Burrowing Crayfish, Engaeus affinis Smith & Schuster, 1913 .
Miscellaneous observations during sampling include: C. latimanus dug from burrows beside a waterbody and C. destructor dip-netted 1.0 m away from within the waterbody at Reedy Lake (west of Nagambie) and at the Rubicon River floodplain at Thornton; C. latimanus captured within 0.5 m of E. curvisuturus Horwitz, 1990 , at the Rubicon River at Thornton, and E. lyelli near Lake Nillahcootie. Further, C. latimanus was dug from a burrow on the bank 0.05 m above the water level of an unnamed creek at Eildon, with C. destructor dug from a burrow 0.15 m further upstream and E. lyelli collected 0.17 m downstream.
Threats. Based on its propensity to spend a large portion of its life in underground burrows connected to the water table, C. latimanus may suffer increased mortality during events, such as supra-seasonal droughts or groundwater extraction. During these times, the water table may disconnect from burrow systems for long durations, and/or the burrows may be exposed during deeper than usual desiccation cracking of the usually moist overlying soil layer. This may be more prevalent in the higher elevation areas away from drainage lines and may not benefit from environmental watering conducted during these times if water volumes are insufficient to inundate these higher zones. Desiccation cracking to a depth of about 2.2 m was noticed near the end of the Millennium Drought on a claypan in the Millewa Forest occupied by C. latimanus ; the cracks extended down to near a chamber of a burrow system.
Recreational fishing pressure may also impact population abundance and recruitment, as C. latimanus can grow larger than C. destructor and is therefore more sought after. Take of both species is currently controlled under recreational fishing regulations in New South Wales and Victoria (as Yabby Cherax spp. — Victoria; Yabby Cherax destructor — New South Wales), though there is no legal minimum size, and large bag and possession limits. These regulations may not be effective in preventing the decline of populations of C. latimanus , particularly given the smaller range of this species compared with that of C. destructor , and the short timeframe that the species is found in high abundance outside of its underground burrows when it can be efficiently harvested.
An additional potential threat may include habitat degradation through cattle pugging along stream banks and moist, seasonal wetlands, though the species has been found in cleared paddocks with stock, and in roadside drains.
Remarks. Cherax latimanus is similar to three other Australian congeners, C. punctatus Clark, 1936 (coastal Qld), C. rotundus (Severn River, MDB, Qld) , and C. setosus (coastal NSW), all displaying similar morphology including large chelae and ventral propodal setation. The new species can be separated from C. punctatus and C. setosus on the basis of many morphological characters, though primarily by the presence of mesial dactylar basal spines (versus 0), two prominent teeth on dactylar cutting edge (versus 1), rough texture of the lateral propodal margin (versus smooth), and a broad, paddle-shaped mesial carpal spine (versus pointed spine) (see Appendix 1 for comparative material examined). Cherax latimanus can be separated from C. rotundus based on morphological differences in the chelae and rostral tip between ( Table 6). Both species are present in the Murray-Darling Basin, albeit with widely separated allopatric distributions, have similar chelae and have been confused in the literature.
Cherax latimanus , included in the molecular phylogenetic analysis of the Cherax destructor complex ( Unmack et al. 2019) as “C. sp. Swamp”, was supported as genetically distinct from C. destructor (C. d. destructor + C. d. albidus ), with which it is found sympatrically, based on well supported phylogenetic analysis on individual SNP genotype and fixed difference analysis (9–27% fixed differences).
| R |
Departamento de Geologia, Universidad de Chile |
| AM |
Australian Museum |
| NMV |
Museum Victoria |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cherax latimanus
| Raadik, Tarmo A. 2021 |
Cherax sp.
| Unmack, P. J. & Young, M. J. & Gruber, B. & White, D. & Kilian, A. & Zhang, X. & Georges, A. 2019: 859 |
Cherax
| Lawrence, C. S. & Morrissy, N. M. & Vercoe, P. E. & Williams, I. H. 2006: 31 |
Cherax rotundus rotundus
| Austin, C. M. & Nguyen, T. T. T. & Meewan, M. M. & Jerry, D. R. 2003: 102 |
Cherax
| Littlejohn, P. 2001: 755 |
Cherax sp.
| Edney, G. N. & McNeil, D. G. & Lawler, S. H. 2002: 200 |
| Lawrence, C. S. & Morrissy, N. M. & Bellanger, J. & Cheng, Y. W. 1998: 100 |
Cherax destructor
| Mussared, D. 1997: 60 |
Cherax diogenes
| Littlejohn, P. 2001: 754 |
| Crabb, P. 1997: 187 |
Cherax sp. C
| Anon 2018: 10 |
| Hale, J. & Butcher, R. 2011: 20 |
| Horwitz, P. & Austin, C. M. 1995: 32 |
Cherax rotundus
| Anon 2017: 1 |
| Bentley, A. I. 2014: 107 |
| Jones, H. A. 2011: 289 |
| Schultz, M. B. & Smith, S. A. & Horwitz, P. & Richardson, A. M. M. & Crandall, K. A. & Austin, C. M. 2009: 585 |
| McCormack, R. B. 2008: 27 |
| Nguyen, T. T. T. & Austin, C. M. 2005: 210 |
| Munasinghe, D. H. N. & Burridge, C. P. & Austin, C. M. 2004: 556 |
| Austin, C. M. & Nguyen, T. T. T. & Meewan, M. M. & Jerry, D. R. 2003: 102 |
| Sokol, A. 1986: 227 |