Assiculoidinae
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3718.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:87B12108-9A55-487B-9C47-48CAA711A9F6 |
|
DOI |
https://doi.org/10.5281/zenodo.6155250 |
|
persistent identifier |
https://treatment.plazi.org/id/D471245E-1369-FFAA-A5A9-FA7E04C52616 |
|
treatment provided by |
Plazi |
|
scientific name |
Assiculoidinae |
| status |
|
Assiculoidinae new subfamily
Type genus. Assiculoides Gill & Hutchins 1997 .
Diagnosis. The Assiculoidinae is cladistically diagnosed by the following three autapomorphies:
6. Anteroventral scale rows on posterior part of body and caudal peduncle oriented almost vertically: In most pseudochromids the scale rows on the posterior part and caudal peduncle are arranged in an obviously anteroventral direction ( Figure 6 View FIGURE 6 A). Uniquely among pseudochromids, in Assiculoides the scale rows on the posterior part of body and caudal peduncle are oriented almost vertically ( Figure 6 View FIGURE 6 B).
7. Dorsal fin connected to caudal fin by membrane: The dorsal fin is completely separate from the caudal fin in Assiculus , pseudochromines, anisochromines and pseudoplesiopines ( Figure 6 View FIGURE 6 A). Assiculoides is derived in having the posteriormost dorsal-fin ray bound posteriorly by a low membrane to the dorsal edge of the caudal fin, the membrane interrupting the circumpeduncular scales so that the median row of scales along the dorsal edge of caudal peduncle is absent ( Figure 6 View FIGURE 6 B). With the exception of Rusichthys , in which the dorsal fin is nonetheless connected to the caudal peduncle by a low membrane, the dorsal fins (and anal fins) of congrogadines are connected to the caudal fin by membrane. Depending on species, the membrane varies from low (connecting to only the base of the final dorsal- and anal-fin rays) to high (with the three fins fully confluent). Such connection is common in eel-like fishes. Consideration of other characters that nest congrogadines within the Pseudochromidae as the sister group of the Anisochrominae ( Figure 1 View FIGURE 1 ) reveals the dorsal-fin connection in congrogadines to be nonhomologous with the condition found in Assiculoides .
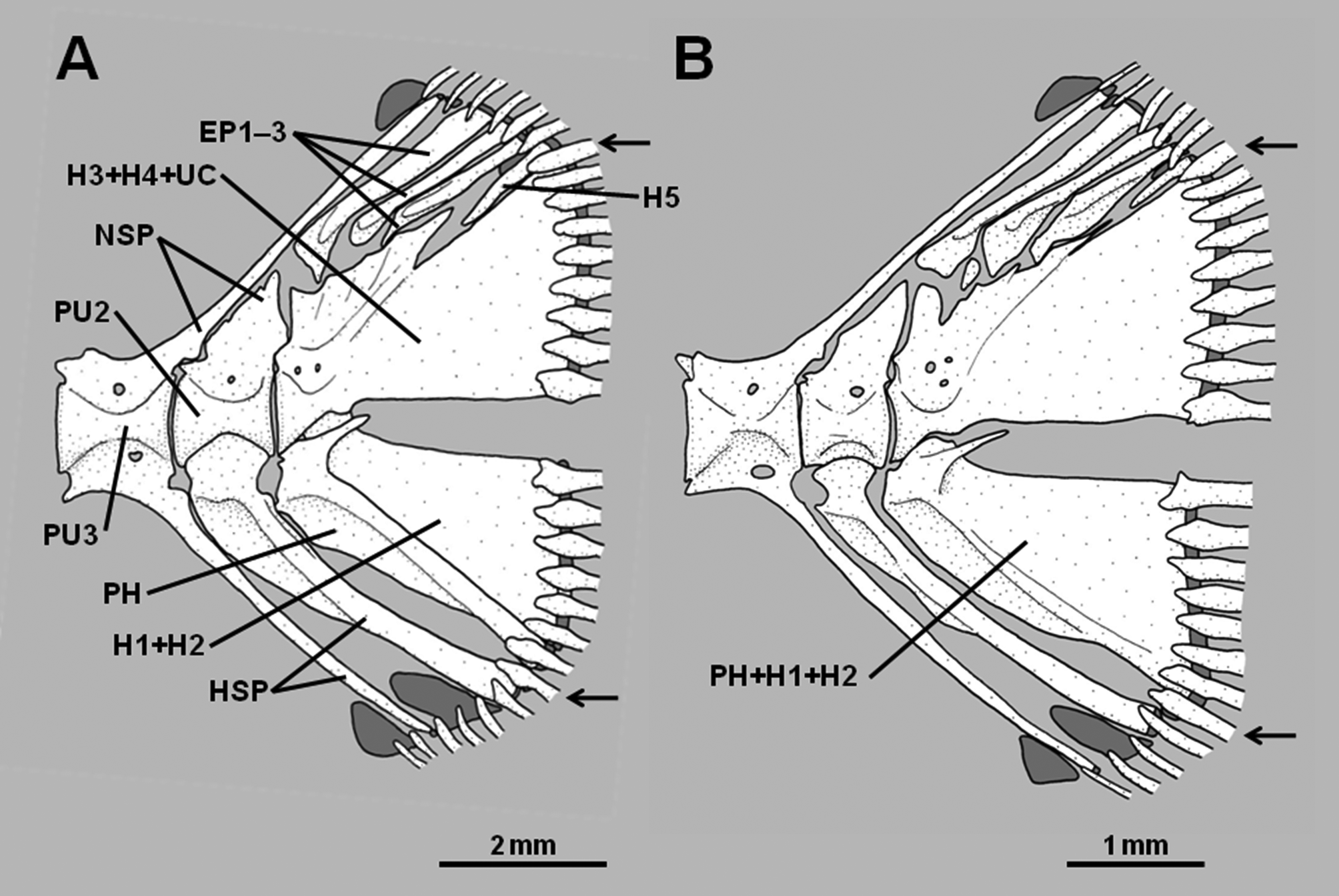
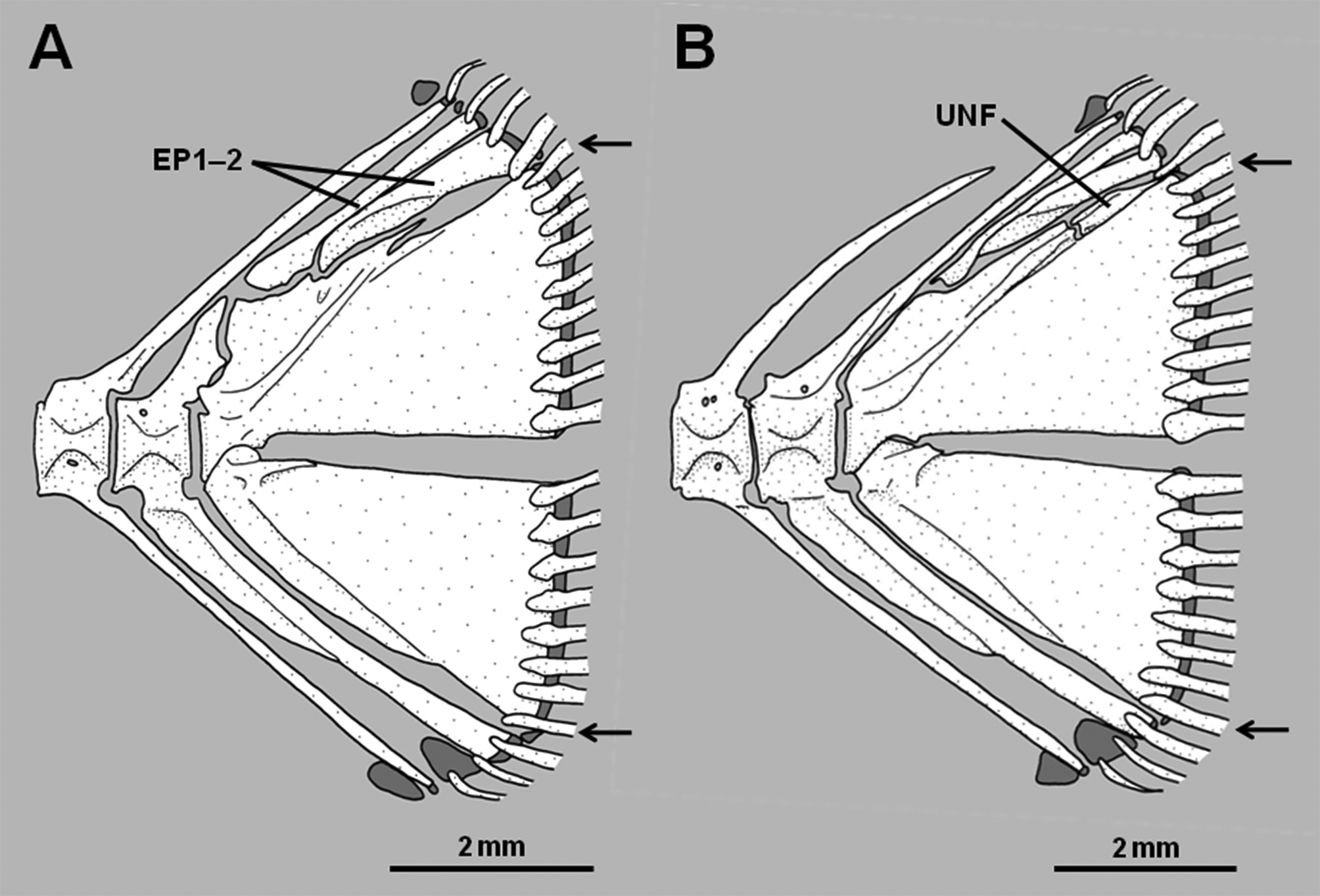
8. Hypural 5 reduced in size or absent: A relatively well-developed hypural 5 is present in pseudochromines, Assiculus , pseudoplesiopines and anisochromines ( Figures 2 View FIGURE 2 A–B, Springer et al. 1977, Gill & Edwards 1999). In Assiculoides it is either present as a tiny splint at the posterodorsal corner of hypurals 3+4, or absent ( Figures 3 View FIGURE 3 A– B; note that the small bone in the approximate position of hypural 5 in Fig. 3 View FIGURE 3 B is actually an anomalous uroneural fragment). Hypural 5 is also absent in all congrogadines (Godkin & Winterbottom 1985, Winterbottom 1996). As for character 7, other characters reveal the absence of hypural 5 in Assiculoides and congrogadines to be independently derived (i.e., a homoplasy).
In addition, the following character combination allows ready identification of the subfamily: dorsal-fin rays III,25–27 (usually III,26); anal-fin rays III,14–16 (usually III,15); pelvic-fin rays I,5, all segmented rays branched; lateral line represented by an anterodorsal and a posterolateral series of tubed scales; caudal peduncle relatively short (5.2–7.9 % SL).
Remarks. Includes only Assiculoides desmonotus Gill & Hutchins 1997 from coastal regions of the Kimberley district of Western Australia. See Gill (2004) for a detailed description of the species.
Assiculoides desmonotus shows marked variation in the development of the pu2 neural spine. Of 58 specimens of A. desmonotus examined (see Gill 2004), 48 (83%) have a short pu2 neural spine that does not extend above the proximal tip of the first epural. The neural spine of the pu3 centrum is cartilage-tipped and long, extending to the dorsal margin of the caudal skeleton, and usually supports the anteriormost procurrent caudal-fin ray. The second epural is much broader than the first. The pu2 haemal spine is not forked distally ( Figure 3 View FIGURE 3 A). The remaining 10 specimens (17% of total) have an elongate, cartilage-tipped pu2 neural spine, which extends to the dorsal margin of the caudal skeleton and usually supports the anteriormost procurrent caudal-fin ray, thus resembling the pu3 neural spine of specimens with a short pu2 neural spine. The neural spine of the pu3 centrum is relatively short, comparable in length to those of more anterior vertebrae, and neither reaches to the dorsal margin of the caudal skeleton nor supports a ray. The second epural is only slightly broader than the first. The pu2 haemal spine is either forked or unforked distally ( Figure 3 View FIGURE 3 B).
Anisochromines display similar variation in pu2 neural spine length. Springer et al. (1977, p. 4) noted that the pu2 neural spine of Anisochromis straussi Springer, Smith & Fraser (1977) is “usually short, occasionally long.” My observations on the type specimens of A. straussi , and on specimens of the remaining two species in the Anisochrominae , A. kenyae Smith (1954) and A. mascarenensis Gill & Fricke (2001) (see Gill & Fricke 2001 for list of specimens examined), revealed the following variation: A. straussi , short in 65 specimens, moderately long (reaching above middle of first epural, but not extending to dorsal edge of caudal skeleton) in four specimens, and “full” in 13 specimens; A. kenyae , short in 39 specimens, moderately long in two specimens, and “full” in four specimens; A. mascarenensis , short in nine specimens, and moderately long in one specimen.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.