Nola estonica Õunap, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5082.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:6B624C07-9C58-4FBE-A2FE-1D16BA9426CA |
|
DOI |
https://doi.org/10.5281/zenodo.5794918 |
|
persistent identifier |
https://treatment.plazi.org/id/BEBFF5BB-9C90-4BD5-8145-6BFEFA367EEF |
|
taxon LSID |
lsid:zoobank.org:act:BEBFF5BB-9C90-4BD5-8145-6BFEFA367EEF |
|
treatment provided by |
Plazi |
|
scientific name |
Nola estonica Õunap |
| status |
sp. nov. |
Nola estonica Õunap sp. nov.
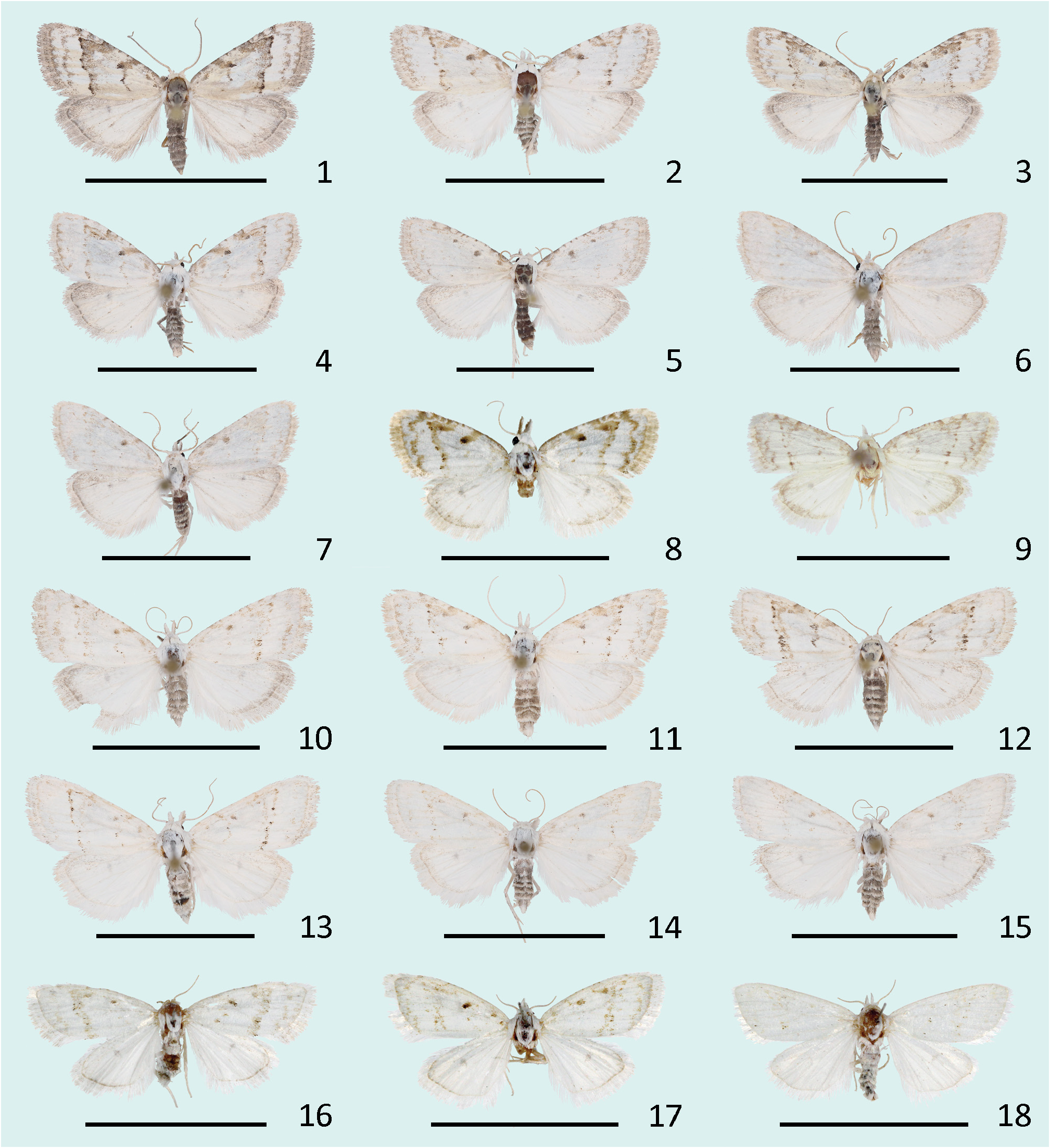
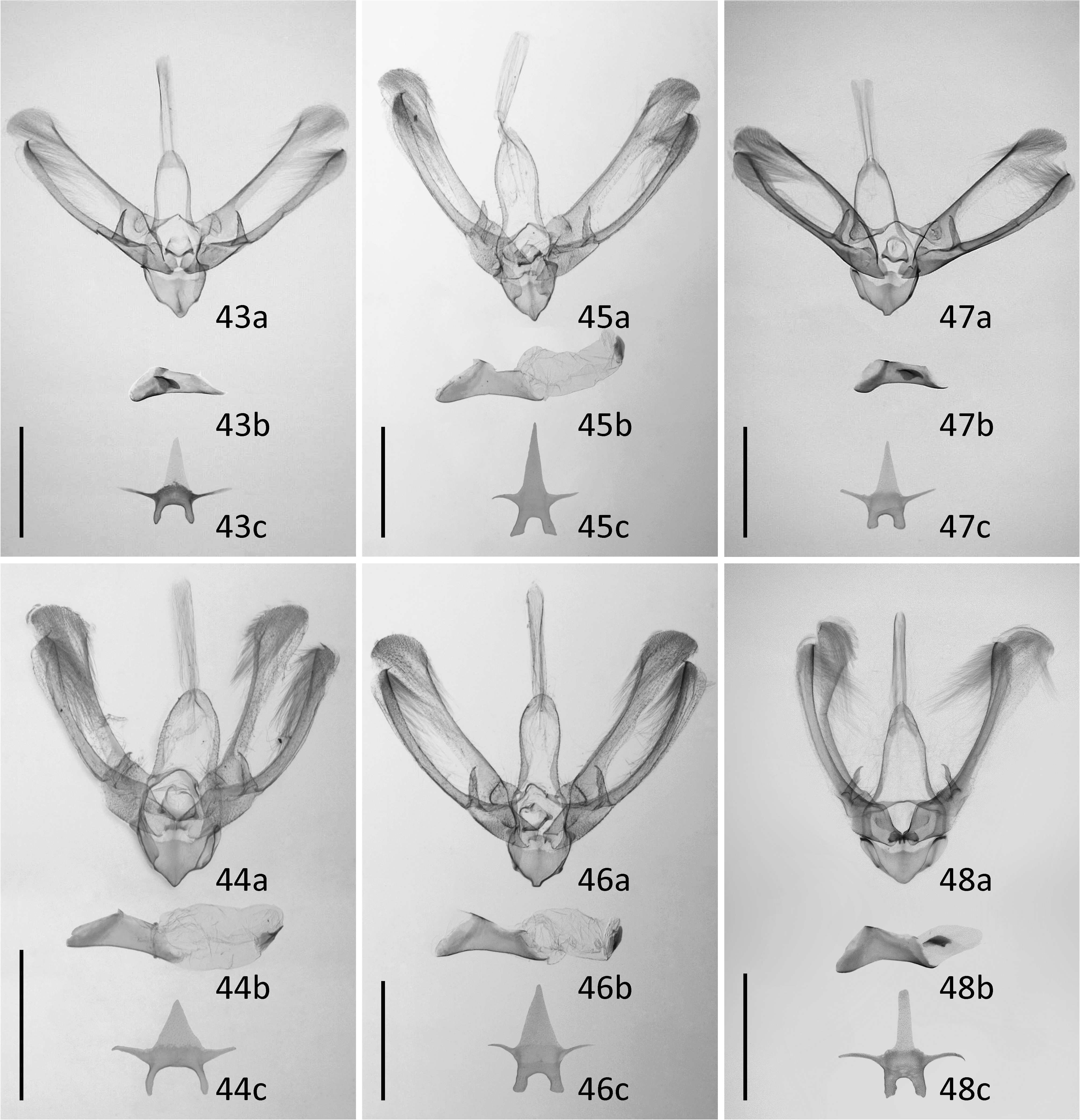
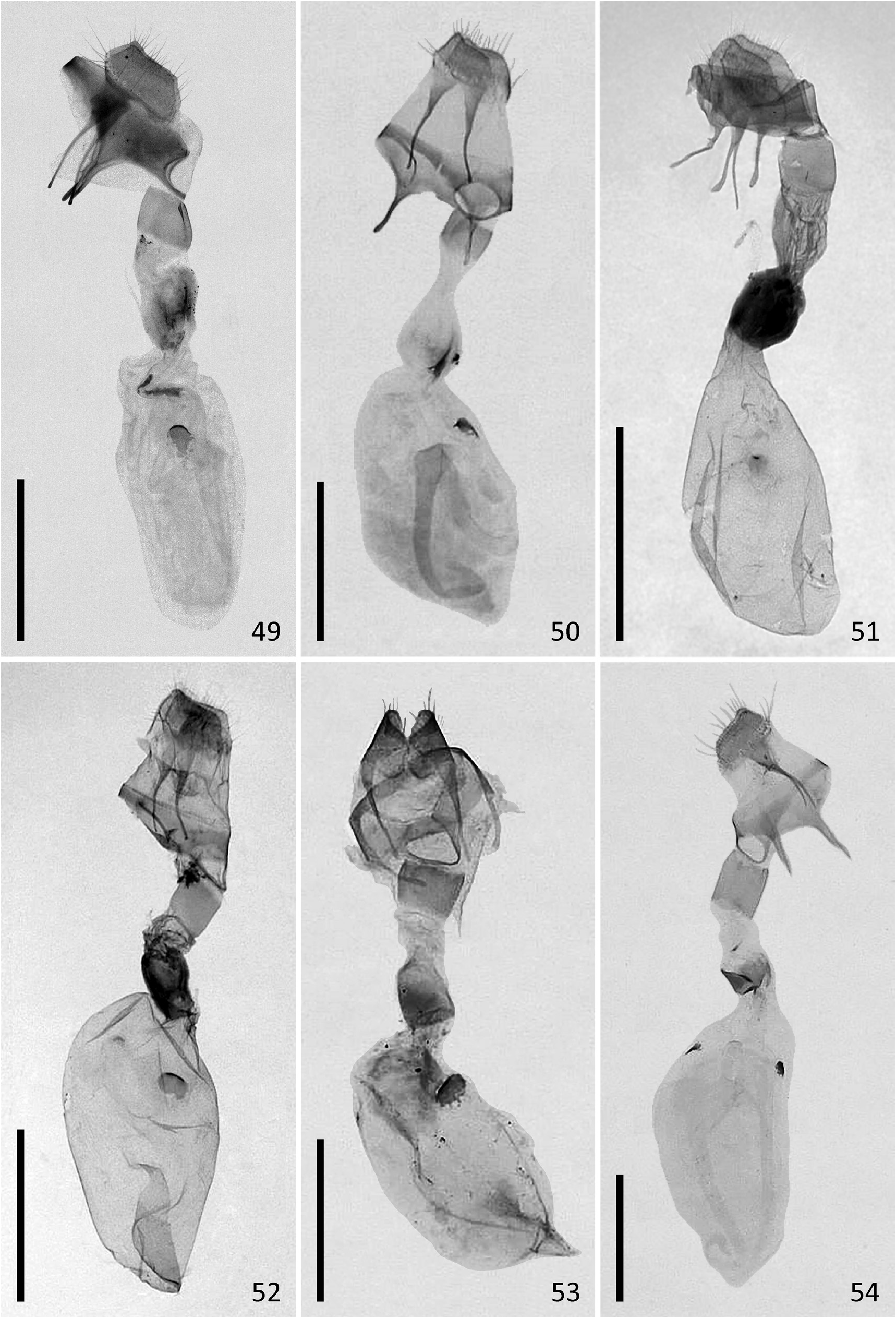
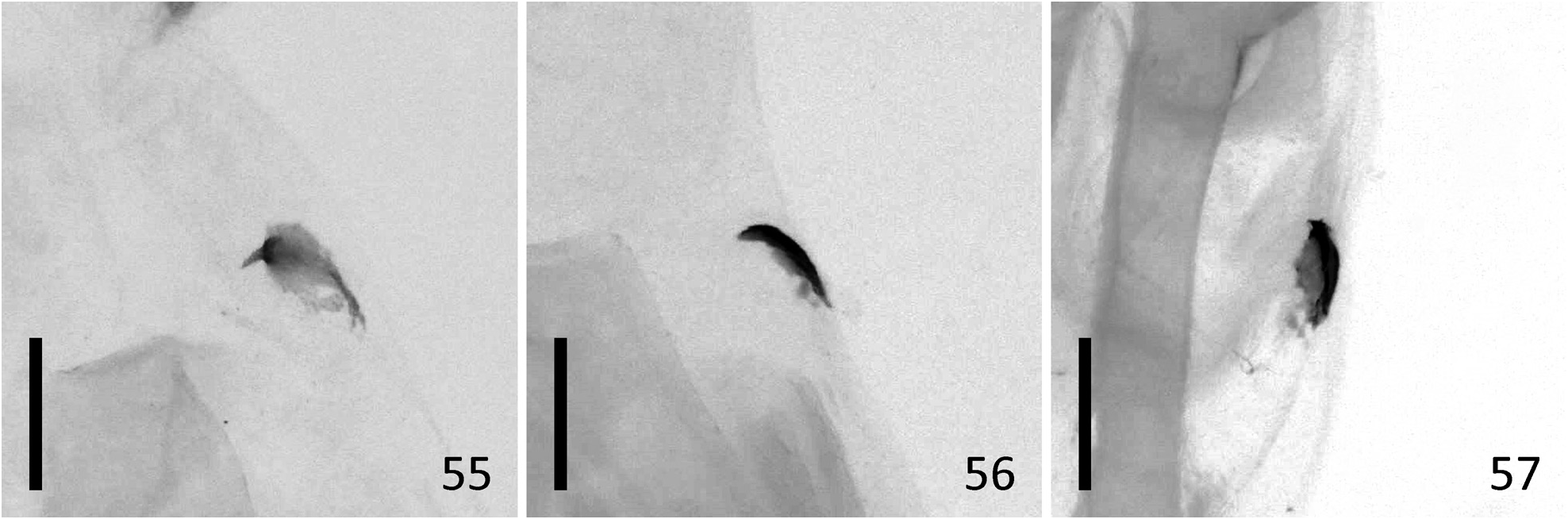
( Figures 1–18 View FIGURES 1–18 , 43–44 View FIGURES 43–48 , 49–51 View FIGURES 49–54 , 55 View FIGURES 55–57 )
Type material
Holotype: ♀, ESTONIA, Piusa Railway Station , at light, 57°50’20.9’’N 27°28’15.0’’E, 03.08.2020, leg. E. Õunap, TUZ300299 . GoogleMaps
Paratypes, 82♂♂, 53♀♀.
ESTONIA
1♂, Põlvamaa, Värska, 57°58’N 27°37’E, 19.09.2001, leg. T. Ruben/A. Lindt, IZBE1137190.
1♂, Põlvamaa, Korela, 57°53’N 27°44’E, 01.- 15.07.2010, leg. T. Ruben, IZBE1137191.
1♂, Värska, Õrsava, 57°56’46“N 27°37’54“E, 19.07.2011, leg. T. Tammaru, DNA voucher EÕ1488, RCTT.
1♀, Piusa Railway Station, 57°50’30’’N 27°27’26’’E, 20.07.2011, leg. T. Tammaru, DNA voucher EÕ1489, RCTT.
1♂, Harjumaa, Mustjõe, 59°19’N 25°28’E, 01.- 19.07.2012, leg. T. Ruben, IZBE1137192.
1♀, Mäe-Palo, 57°37’06’’N 27°07’26.5’’E, 04.07.2012, leg. E. Õunap, DNA voucher EÕ1490, RCEÕ.
1♂, Piusa, 57°50’30’’N 27°27’18’’E, 03.08.2017, leg. I. Taal & T. Tasane, RCIT.
1♂, Karilatsi 1 km W, 58°07’25’’N 26°54’05’’E, 07.09.2018, leg. T. Tammaru, DNA voucher EÕ1484, RCTT.
1♂, Parmu, at light, 57°33′53.3″N 27°19′15.4″E, 20.07.2020, leg. E. Õunap, RCEÕ.
6♂♂, 2♀♀, Piusa Railway Station, 57°50’21’’N 27°28’14’’E, 27.07.2020, leg. I. Taal & A. Truuverk (incl. 2♂♂, DNA vouchers EÕ1550, EÕ1552, used for genetic study), RCIT.
5♂♂, 5♀♀, Piusa Railway Station, 57°50’21’’N 27°28’14’’E, 27.07.2020, leg. I. Taal & A. Truuverk, RCAT.
48♂♂, 30♀♀, Piusa Railway Station, 57°50’21’’N 27°28’14’’E, 27.07.2020, leg. I. Taal & A. Truuverk, 2♂♂, 2♀♀ dissected, TUZ300207–TUZ300284.
3♂♂, 3♀♀, Piusa Railway Station, at light, 57°50’20.9’’N 27°28’15.0’’E, 03.08.2020, leg. E. Õunap (incl. 1♂, DNA voucher EÕ1529, used for genetic study) RCEÕ.
13♂♂, 11♀♀, Piusa Railway Station, at light, 57°50’20.9’’N 27°28’15.0’’E, 03.08.2020, leg. E. Õunap, 2♂♂, 5♀♀ dissected, TUZ300285–TUZ300298, TUZ300300–TUZ300309.
Other material examined
RUSSIA
1♀, Primorsky region, Kedrovaja Pad, V, L[ight], 43°06’N 131°29’E, 2- 17.08.1997, leg. Laanetu & Viidalepp, dissected, IZBE0106558.
1♂, Amurskaja region, Svobodnenski district, Iverskii zakaznik, 18.06.- 01.07.2010, leg. A. Barbarich, A. Streltsov, P. Osipov, dissected, slide Matov 0589, ZISP.
SOUTH KOREA
6♀♀, Mt. Samaksan, Deokduwon-ri, Seo-myon, Chuncheon, Gangwon-do Province, at light, 37°50’11’’N 127°37’30’’E, 25.06.2016, leg. S. S. Kim, 2 ♀♀ dissected, MNU genital slides no. 1172 and 1173, MNU 5- MNU 10.
1♂ Haesan, Hwacheon-gun, Gangwon-do Province, at light, 38°11'15’’N 127°47'18’’E, 24.06.2017, leg. S. S. Kim, dissected, MNU genital slide no. 1170, MNU NE1.
Description
External morphology. Wingspan 15.2-18.1 (average 16.4 ± 1.0 SD, n = 18) mm in males 15.4-19.0 (average 17.2 ± 1.0 SD, n = 16) mm in females. Head white, antennae covered with white scales. Male antennae bipectinate, bearing numerous sensilla on the ventral side. The length of sensilla exceed the diameter of the flagellum. Female antennae filiform. Labial palpi porrect, elongated, more than two times longer than the diameter of the eye, intermixed with light and dark scales on the lateral side, but only white scales present on the medial side. Proboscis present. Thorax white. Forewing elongated, apex rounded. Upperside white. Three tufts of raised scales present along the anterior edge of the cell, the medial and distal tuft always containing at least some dark scales, the proximal tuft sometimes completely white. Subbasal line present as a brown costal blotch, sometimes completely absent. Antemedial line, if present, usually brown, rarely black, jagged, forming an irregular curve towards the termen. A large brown blotch sometimes present on costa proximal to the antemedial line. Medial line absent. Postmedial line brown, rarely black, parallel to costa in the subcostal region, but turns towards inner margin at an acute angle on R 5. Postmedial line almost straight between R 5 and inner margin, with clear darker spots on veins, sometimes proximally accompanied by a light brown band. Subterminal line undulating, light brown to light grey, sometimes completely absent. Terminal line light brown to light grey, sometimes hardly visible, sometimes interrupted by a row of white or yellowish dots on veins. Fringes usually unicolourous, white, light beige or light grey, rarely slightly lighter on veins. Pattern reduced in many specimens, sometimes represented only by a few dark scales on subcostal hair tufts, and as a row of small dark dots referring to postmedial line. Underside unicolourous dark grey in males, white with most veins dark grey and some grey scales diffused between the veins in females. Hindwing with evenly curved termen, apex rounded. Upperside white, subcostal region light grey. In darker specimens wings gradually darkening from white to light grey in subterminal area. Discal spot very weak, formed by a small number of dark scales. Terminal line light grey, interrupted by a row of white or yellowish dots on veins, sometimes hardly visible. Fringes white, light beige or light grey. Underside white, with diffused grey scales mostly present on the anterior half of the wing and on the subterminal area. Discal spot grey. Legs white or grey, darker in males than in females, one pair of tibial spurs present in midlegs, and two pairs in hindlegs of both sexes. Abdomen dorsally light yellowish grey, posterior edges of segments visible as a row of lighter scales. Ventral side of the abdomen light yellowish grey suffused with small number of black scales.
Male genitalia. Uncus absent. Tegumen narrow, 1.5 times longer than vinculum. Saccus short and very wide, with rounded tip. Scaphium with two extremely long, parallel, stick-like, sclerotized structures. Valva long, bilobed, costa and ventral margin heavily sclerotized, rounded at both tips. Tip of the ventral lobe of valva extended to a tiny hook. Harpe strong, triangular, spine-like, with a pointed tip. Editum present as a rounded protuberance bearing a number of tiny papilles carrying thin setae, positioned close to base of costa. Transtilla narrow, heavily sclerotized. Juxta plate-like, laterally extended as two arms to dorsal side. Aedeagus almost straight, three times longer than wide, apex ventrally elongated as a thin triangular slat, coecum absent. Vesica straight, slightly wider and longer than aedeagus, with one cornutus. Cornutus short and wide, with a prominent central ridge extending beyond its posterior edge. Eighth tergite with two narrow anterior projections located wide apart from each other, posterior edge of the heavily sclerotized area rounded.
Female genitalia. Ovipositor short, very wide; posterior apophyses approximately as long as ovipositor. Anterior apophyses short, their length approximately 2/3 of the length of posterior apophyses. Ostium bursae heavily sclerotized, genital orifice oval, wider than long. Antrum region very short, membranous. Posterior part of ductus bursae moderately sclerotized, the sclerotized region wider than long, its length about 1/5 of the total length of ductus bursae. Middle part of ductus bursae membranous, two times longer than wide, the membrane slightly wrinkled, sometimes with irregular patches of sclerotization. Anterior part of ductus bursae heavily sclerotized, dilated, sclerotization present as irregular longitudinal folds. Corpus bursae ovoid, elongate, 2.5 times longer than wide, with one signum. The posterior part of signum bursae bearing a heavily sclerotized thorn pointing towards the lumen of corpus bursae.
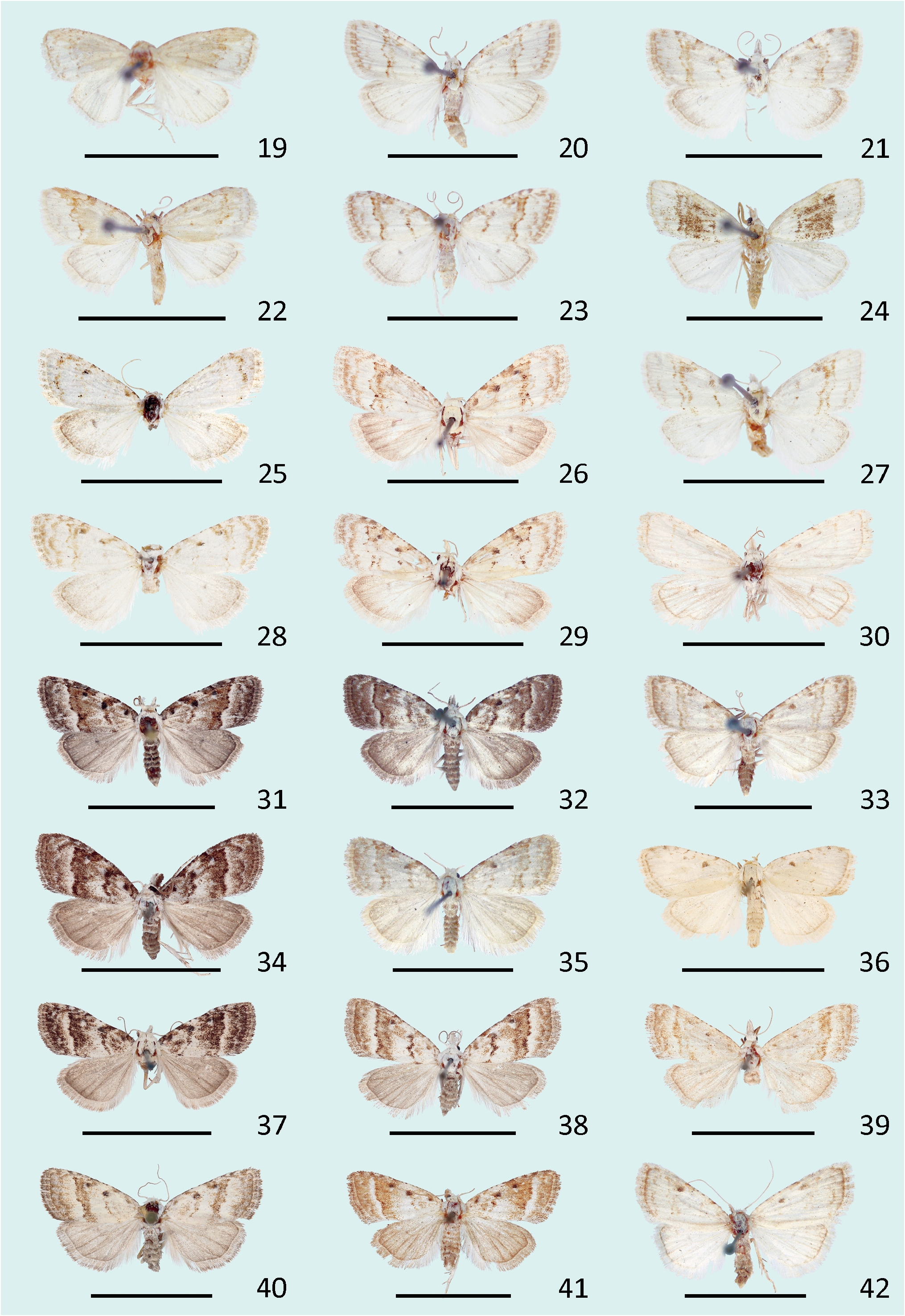
Diagnosis. N. estonica ( Figures 1–18 View FIGURES 1–18 ) differs from N. atomosa by its rather straight postmedial line which is darker on veins and often divided into a row of dark spots. The postmedial line of N. atomosa ( Figures 19–30 View FIGURES 19–42 ) is strongly undulating and almost unicolourous. Even in very light specimens of N. atomosa the postmedial line is not interrupted into separate spots located on veins. In N. atomosa , fringes are chequered, being white on tips of the veins, and light grey between the veins. Male genitalia of N. estonica ( Figures 43 View FIGURES 43–48 ab, 44ab) and N. atomosa ( Figures 45 View FIGURES 43–48 ab, 46ab) are very similar and cannot be used for reliable identification. However, the 8th tergite of N. estonica has narrow anterior projections that are situated apart from each other ( Figures 43c, 44c View FIGURES 43–48 ), while that of N. atomosa usually has wide anterior projections that are located much closer to each other ( Figures 45c, 46c View FIGURES 43–48 ). Females of N. estonica can easily be separated from N. atomosa by genitalia dissection, as this species has only one signum in bursa copulatrix, which is located ventrolaterally ( Figures 49–51 View FIGURES 49–54 ). N. atomosa has an additional smaller signum on the opposite side of bursa copulatrix ( Figures 52–53 View FIGURES 49–54 ), though the latter may be small, almost transparent and therefore hard to notice. A fine detail characteristic of N. estonica is an inward-pointing thorn on the posterior edge of signum ( Figure 55 View FIGURES 55–57 ). Though the posterior edge of the larger signum of N. atomosa is also bent inwards ( Figure 56 View FIGURES 55–57 ), it does not form a distinct narrow thorn. The sclerotized posterior part of ductus bursae is wider than long in N. estonica , but almost rectangular in N. atomosa .
N. aerugula ( Figures 31–42 View FIGURES 19–42 ) can usually be separated from N. estonica by its much darker colouration. Even in very light specimens of N. aerugula the ground colour of forewings is often yellowish, not white, as opposed to the pure white ground colour of N. estonica . Though the postmedial line of N. aerugula is sometimes almost as straight as that of N. estonica , it is not distinctly darker on veins nor divided into a row of spots. The hindwings of N. aerugula are almost unicolourous and darker than those of N. estonica : dark grey in the darkest specimens, light grey in the lightest ones. Male genitalia of N. aerugula ( Figures 47a, 48a View FIGURES 43–48 ) differ from those of N. estonica ( Figures 43a, 44a View FIGURES 43–48 ) by shorter vinculum, which has length/width ratio of about 0.5 (as opposed to at least 0.6 in N. estonica ), and by very short and narrow saccus. There are, however, no differences in the shape of the aedeagus of N. estonica ( Figures 43b, 44b View FIGURES 43–48 ) and N. aerugula ( Figures 47b, 48b View FIGURES 43–48 ). The 8th tergite of N. estonica has narrow anterior projections that are situated apart from each other ( Figures 43c, 44c View FIGURES 43–48 ), while that of N. aerugula usually has wide anterior projections that are located much closer to each other ( Figures 47c, 48c View FIGURES 43–48 ). Females of N. estonica can easily be separated from N. aerugula by genitalia dissection, as this species has only one ventrolateral signum on bursa copulatrix ( Figures 49–51 View FIGURES 49–54 ), but N. aerugula has an additional smaller signum on the opposite side of bursa copulatrix ( Figure 54 View FIGURES 49–54 ). However, the latter may be small, almost transparent and therefore hard to notice. The larger signum of N. aerugula is often just a flat patch of sclerotization on the wall of bursa copulatrix which is thicker on its posterior edge ( Figure 57 View FIGURES 55–57 ), but sometimes its posterior edge is bent inwards. Even in the latter case it does not form a distinct narrow inward-pointing thorn which is characteristic to N. estonica . The sclerotized posterior part of ductus bursae is wider than long in N. estonica , but almost rectangular in N. aerugula .
Note. Though the hitherto known European and Far Eastern populations of N. estonica are separated by at least 6000 kilometers, we have not found any consistent differences in their morphology. The South Korean and Russian specimens fit well within the intraspecific variation of the Estonian material.
Biology. N. estonica appears to be locally common in southeastern Estonia. The majority of the type series were collected from a dry, narrow meadow stripe in the railway corridor that penetrates a landscape dominated by dry pine forest on sandy soil. Whether the species prefers woodland or open habitat is yet unknown, as though the moths were captured on a meadow, they may have flown to light from the nearby forest only 15-20 meters away. In South Korea, the moths were collected in mountainous woodland with mixed coniferous and deciduous trees, and the single contemporary specimen from Russian Far East was taken from mixed forest adjacent to large xerophytic meadows. Most of the hitherto known specimens have been collected in July and early August, but two records from September suggest that partial second brood may exist. Other details of the life cycle and larval foodplants are not known.
Etymology. The name estonica refers to Estonia, as the species was first discovered in this country, which is also the area of origin of the type series.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Nolinae |
|
Genus |