Gammarus alpinus, Roman Alther, Cene Fišer & Florian Altermatt, 2016
|
publication ID |
https://doi.org/ 10.1111/zoj.12477 |
|
DOI |
https://doi.org/10.5281/zenodo.6055336 |
|
persistent identifier |
https://treatment.plazi.org/id/CF213B4D-7E3C-FFB5-FC49-FE13FB00DB2E |
|
treatment provided by |
Plazi |
|
scientific name |
Gammarus alpinus |
| status |
sp. nov. |
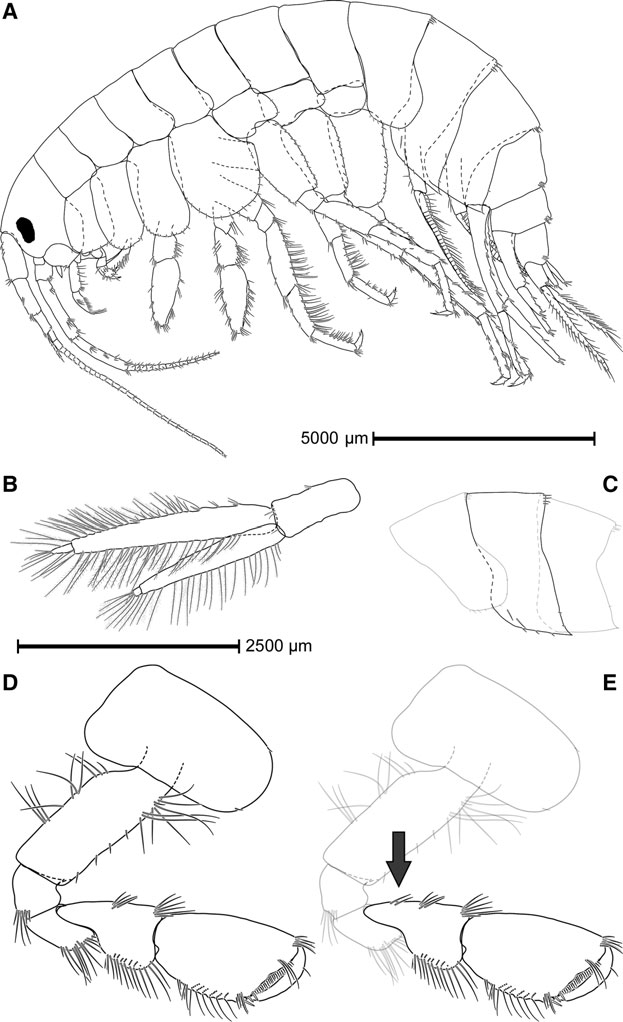
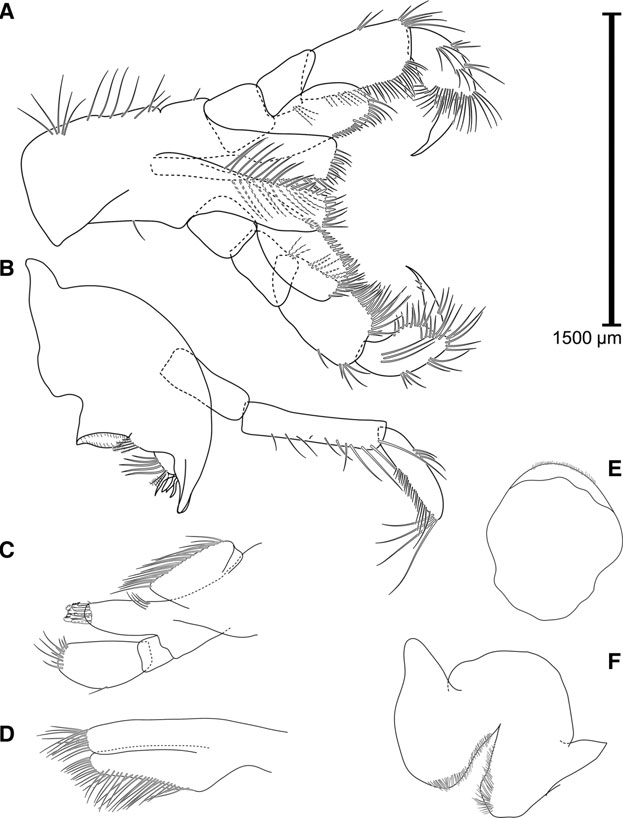
GAMMARUS ALPINUS View in CoL SP. NOV. ( FIGS 5 View Figure 5 , 6 View Figure 6 )
References (that now most likely have to be considered G. alpinus View in CoL sp. nov.)
1, 2, 8, 12, 14 – 16, 19, 22, 35 – 38, 45, 46, 49, 52 – 54, 65, 66, 72, 73, 83 – 86, 94, 101 and 102 in Supporting Information (Table S1).
Type locality
Lej da San Murezzan (Lake St. Moritz) , Switzerland, 9°50 ′ 51.05 ″ E, 46°29 ′ 48.02 ″ N [WGS84], equivalent to 784901 m E, 152323 m N [ CH1903/LV03 ]. GoogleMaps
Material examined
Seventy-seven specimens of G. alpinus in detail (the same were used for molecular analysis), another ~30 when studying specific characters.
Holotype
The male holotype is deposited in the collection of the Musée de Zoologie, Lausanne, Switzerland (voucher number GBIFCH00329236), along with four paratypes (2♂; GBIFCH00329237 & GBIFCH00329 238, 2♀; GBIFCH00329239 & GBIFCH00329240) from the same location, collected by R. Alther, 27.VIII.2014. Ten additional paratypes (5♂, 5♀) from Lej da Silvaplauna (9°47026.97 E, 46°27017.89 N), very close to the type locality, are deposited in our own collection at Eawag, Dübendorf. Additionally, DNA vouchers of analysed specimens are deposited in our own collection.
Etymology
The species epithet, alpinus , refers to its distribution throughout the European Alps, its probable endemic status there and its habitat preference for alpine lakes.
Diagnosis
Morphologically nearly identical to G. lacustris , it is a comparably large and robust species ( Fig. 5 View Figure 5 A). Gammarus alpinus has only one clear group of two to six setae (and very rarely an isolated seta) along the anterior margin of the carpopodite of gnathopod 2 ( Fig. 5 View Figure 5 D), while G. lacustris has two distinct groups that harbour five to eight setae in total ( Fig. 5 View Figure 5 E).
Description
Body length: maximum length (TOT, see Supporting Information, Table S5) within 77 individuals from 18 localities (Supporting Information, Fig. S3) is 20 mm (♀, Lac de Roy, France). Adding the length of first antenna results in more than 32 mm; maximum length of tip of rostrum to tip of third uropod is 25 mm. Females slightly but significantly larger than males (based on a Welch two sample t -test, lengths with and without first antenna; mean length of ♀♀ = 22.17 and 15.00 mm; mean length of ♂♂ = 20.07 and 13.68 mm; P = 0.043 and 0.036, respectively).
Head: eyes roundish or suboval, bean-like form widely found in the genus Gammarus ; lateral cephalic lobe subrounded.
Antenna 1: seldom much longer than one-third of total body length (TOT + antenna 1), never half of it; setation relatively short; main and accessory flagella with 12 – 33 and 2 – 3 (up to 5) segments, respectively; length ratio of peduncular segments 1:2:3 = 1:0.83:0.53; peduncular segments 2 and 3 usually with one tuft of setae each.
Antenna 2: slightly longer than half of the first antenna; prominent and pointy tip of the glandular cone reaches peduncular segment 4; peduncular segments 4 and 5 armed with tufts of setae, usually arranged in three longitudinal rows; short and slender flagellum (4 – 16 segments, usually about 10) never with flag-like brush or swollen appearance; calceoli may be present but not prevalent and no apparent pattern for presence or absence has been found.
Mandibular palp: third segment ( Fig. 6 View Figure 6 B) armed with one group of A-setae, one or two groups of Bsetae, 18 – 30 D-setae and 4 – 5 E-setae.
Metasome: segments 2 and 3 ( Fig. 5 View Figure 5 C) can harbour some small setules along their posterior margins.
Urosome: even, without excavations or elevations.
Gnathopod 1: propodus piriform in both sexes; palmar margin with a single but strong medial spine; 3 – 12 angular spines along inferior margin of the propodus.
Gnathopod 2 ( Fig. 5 View Figure 5 D): propodus with a more rectangular form; no prominent sexual dimorphism in shape; almost exclusively only a single group of setae present along anterior margin of carpopodite (exceptions in 2.6% or two out of 77 individuals), harbouring 2 – 5 setae (one specimen had 6).
Pereiopods: armature comparable to G. lacustris ; no enlarged or prominent posteroinferior lobe in basis of pereiopods 5, 6 and 7; dactylus of P3 – P7 distinctively more slender than in most other members of the the Gammarus pulex group (sensu Karaman & Pinkster, 1977b).
Epimeral plates: posteroinferior corner of the first epimeral plate somewhat rounded, with a merely pointed and retracted edge, harbouring one seta; posteroinferior corner of second and third plate always sharply pointed ( Fig. 5 View Figure 5 C); inferior margin of the epimeral plates harbours setae and often spines, with highly variable absolute number and ratio of spines to setae; often setae or spines on the lateral surface of the second epimeral plate; few short setae may be implanted along the posterior margin of the last two epimeral plates, spines were never recorded; number of spines and setae usually differs between left and right side of the individual.
Uropod 3: endopoditenearly reaches length of the first exopodal segment ( Fig. 5 View Figure 5 B); second exopodite segment usually well developed; proportion of inner to outer ramus between three-quarters and 1; setae along inner and outer margins in both endo- and exopodite virtually always plumose.
Telson: lobes sparsely armed, usually with no more than one or two terminal spines and some setae, most of them usually at least as long as the spines; sometimes a subbasal spine can be found on the surface of the telson lobe in large individuals of both sexes.
Overall habitus: colour of live specimens is greyish to brown or rather white, depending on habitat in which they are found. Specimens can be covered with algae, especially around tufts of setae, depending on habitat.
Molecular diagnosis: affiliation to BIN BOLD: ACH6111 or BOLD:ADB3370 (http://www.boldsys tems.org) corresponds to belonging to Gammarus alpinus . In detail, within the aligned sequences of COI (658 bp, 5 ′ -GACATTATATTTTGTTTTAG-...-CGTTTT AGCCGGAGCTATCA-3 ′) we identified 1 4 SNPs, which are diagnostic for the alpine clade compared to the original G. lacustris (positions 103, 160, 163, 187, 211, 220, 274, 337, 343, 347, 370, 448, 523, 553 in 5 ′– 3 ′ reading direction). One SNP at site 337 even allows the distinction between all three clades. Nucleotide ‘T’ indicates the initial G. lacustris , whereas ‘A’ indicates the southeastern alpine clade and ‘C’ indicates the north-eastern alpine clade.
Variability
The pattern of variability observed in G. alpinus reveals no significant differences among populations and the variability present is associated with postembryonic developmental changes as well as sexual differences.
Distribution
The species is restricted to the European Alps and has been recorded in Austria, France, Germany, Italy, Slovenia and Switzerland. Its possible distribution in the Balkans and Apennines ( Pljakic, 1952; Pljakić, 1963; Iannilli & Ruffo, 2002) remains to be confirmed.
Remarks and affinities
The morphological distinction from its sister species, G. lacustris , comes down to two stable quantitative characters and one qualitative character. One is the absence of a second group of setae (often as a single seta) along the anterior margin of the carpopodite of gnathopod 2 (present in G. alpinus ). The second is that the anterior group of these setae harboured five or fewer setae (a single specimen was as exception), whereas in G. lacustris all studied specimens had at least five setae implanted ( Fig. 5 View Figure 5 E) when summing up both setae groups (Supporting Information, Fig. S4). Additionally, the number of D-setae on the third segment of the mandibular palp seems to be slightly lower in G. alpinus , but without a clear cut-off allowing a reliable distinction. Whereas the structure of the epimeral plates is still a valid and strong discriminating character of the G. lacustris complex from other species of the Gammarus pulex -group, this character cannot be used in the discrimination of G. lacustris from G. alpinus . We thus suggest a distinction based on a barcoding approach, by extracting DNA from a pereiopod, applying the COI-barcoding protocol provided in this paper and aligning the resulting sequence to our published sequences on BOLD (BIN BOLD:ACH6111 or BOLD:ADB3370). Furthermore, we reason that individuals from France, Switzerland or southern Germany that would have been identified as G. lacustris until recently can be assigned to G. alpinus with a high degree of certainty.
Ecology
Gammarus alpinus has a bimodal elevational distribution ( Fig. 7 View Figure 7 ). It predominantly inhabits alpine lakes above 1500 m a.s.l. but also inhabits lowland lakes at around 300 – 500 m a.s.l. in close vicinity to the Alps. These two habitat types do not correspond to the molecular clades found in G. alpinus . The species has never been recorded in headwater streams. A few specimens were found downstream of Lake Constance as well as in small tributaries to Lake Constance close to the inlets ( Altermatt et al., 2016). Whereas the first observation can be explained by passive drift, the findings in the inlets clearly suggest some ability to persist in slow running and small streams and creeks. Gammarus alpinus was often found to hide in conifer debris (R.A. & F.A., pers. observa.). It can persist at very low temperatures close to 0 °C for a long time (findings in ice-sealed lakes).
Zoological nomenclature
In accordance with the ICZN Code of Nomenclature the new species name and status are registered in the Official Register of Zoological Nomenclature (ZooBank). ZooBank Life Science Identifier (LSID) for new species. http://zoobank.org/urn:lsid:zoobank.org: act:C1A6A35D-6F5E-4BD9-98D8-33AF8917C816.
DISCUSSION
A NEW SPECIES AND ITS DISTRIBUTION
We provide the first overview of the G. lacustris complex in the European Alps. The clear pattern of a highly divergent lineage compared to the circumboreal G. lacustris s.s. is surprising, given the wellknown occurrence in the Alps. We raise the alpine populations to species level based on consistent and stable morphological and molecular support, as well as by the clear geographical separation from its sister species. We consider that this separate status is stable and is strongly justified according to the concepts reviewed by De Queiroz (2007).
The newly described G. alpinus is probably endemic to the Alps, and does not overlap in its range with G. lacustris . Further analysis is needed to determine its distribution in Italy and Austria. Particular focus should be laid on the reported Apenninian populations of G. lacustris ( Iannilli & Ruffo, 2002) . As they were described as genetically similar to alpine specimens, we suspect that they are representatives of G. alpinus as well. Sequences from work by Meyran & Taberlet (1998) could not be directly included as their COI fragments were generated with different markers (COI a-H and COI-Gf) and the sequences do not match with the standard Folmer region. On a qualitative level, however, their findings of diverging lineages within the French populations are consistent with our overall findings. Whereas sequences from the northern French Alps match closely together with the Swiss sequences, sequences from the southern French Alps are slightly diverging and match better to sequences from the northern Austrian Alps.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |