Aberrantidrilus Martin, 2015
|
publication ID |
https://doi.org/10.5252/z2015n4a2 |
|
publication LSID |
urn:lsid:zoobank.org:pub:1CDE356F-BEF2-4888-8580-7AB600A97E2B |
|
DOI |
https://doi.org/10.5281/zenodo.14872622 |
|
persistent identifier |
https://treatment.plazi.org/id/C68B563A-1214-4087-B9E3-39F1479C8A2C |
|
taxon LSID |
lsid:zoobank.org:act:C68B563A-1214-4087-B9E3-39F1479C8A2C |
|
treatment provided by |
Felipe |
|
scientific name |
Aberrantidrilus Martin |
| status |
gen. nov. |
Aberrantidrilus Martin , n. gen.
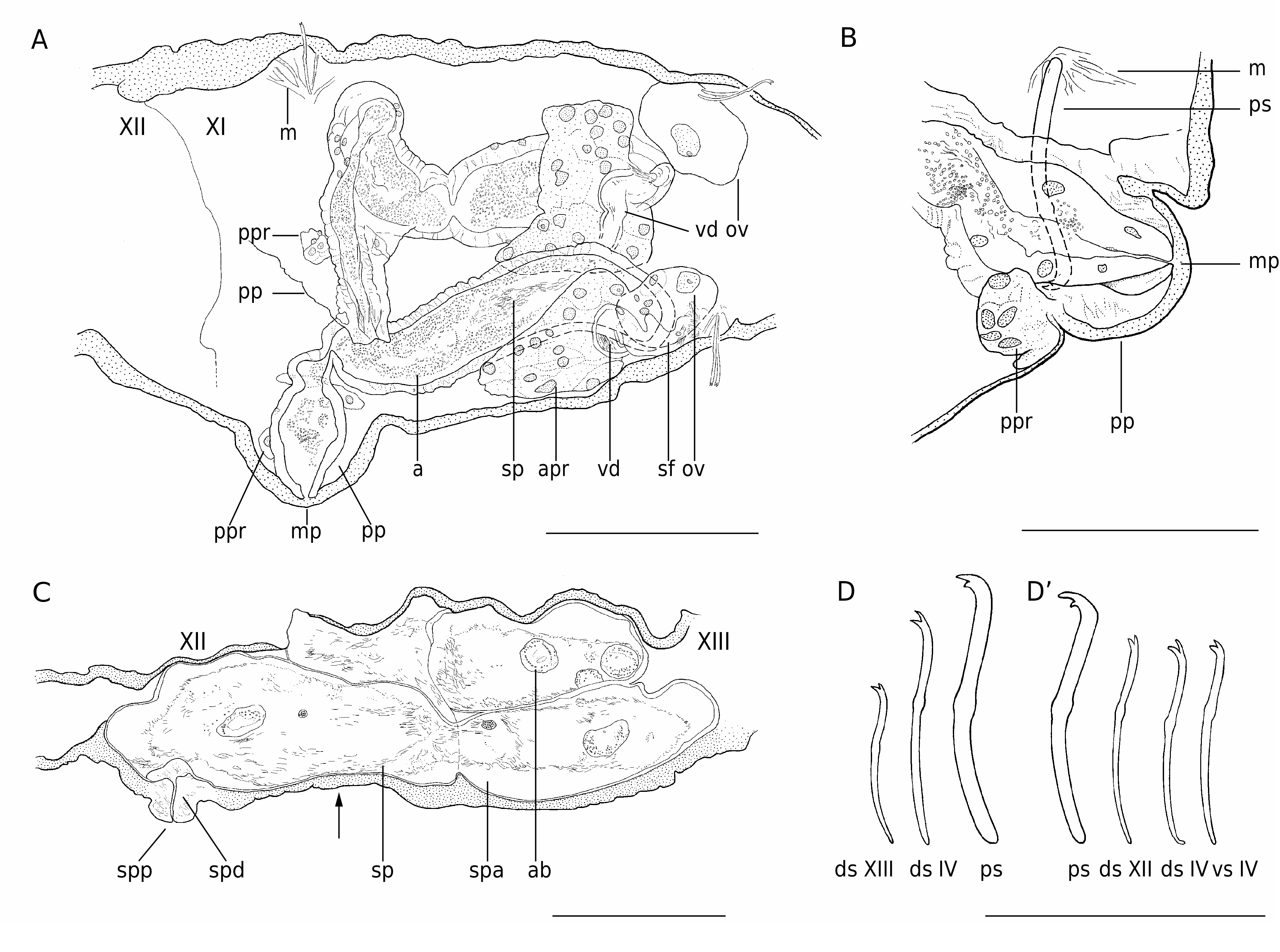
( Fig. 2 View FIG )
TYPE SPECIES. — Aberrantidrilus stephaniae Martin , n. sp. ( Fig. 2 View FIG )
OTHER INCLUDED SPECIES. — Aberrantidrilus cuspis ( Erséus & Dumnicka, 1988) n. comb. and A. subterraneus ( Rodriguez & Giani, 1989) n. comb.
DIAGNOSIS. — Freshwater subterranean phallodrilines. Somatic setae all bifid, similar in all bundles, or with upper tooth much reduced or absent in posterior bundles. Penial setae present, bifid or singlepointed, one per bundle, orientated in anterior direction. Atria covered by a thick muscular layer, opening to exterior on conical male protuberances (porophores). Posterior prostate gland small, attached by a short stalk to proximal end of atrium, at base of pseudopenis. Spermathecal pores in segment XII, on the ventral portion of the body; spermathecae with short ducts and thin-walled ampullae.
ETYMOLOGY. — The name Aberrantidrilus (from drilos, “worm” in Greek and aberrans, -tis, present active participle of the Latin aberrare, “wander, stray, deviating from”) refers to the aberrant location of the spermathecal pores of the species, in segment XII. Spermathecae are in X (or at least anterior to the atrial segment) in all tubificids except one other phallodriline, Mexidrilus postspermathecus ( Erséus 1980) .
REMARKS
The decision to erect a new genus to accommodate all the freshwater subterranean species formerly attributed to Abyssidrilus Erséus, 1992 is supported by a unique combination of characters, rather than formally proposed apomorphies, namely the location of spermathecal pores in XII, the unisetal condition of penial setae and their teeth directed anteriad. The fact that the three species included in Aberrantidrilus Martin , n. gen. are found in subterranean freshwater, as opposed to the marine Abyssidrilus , is considered as additional evidence to support this decision.
The genus Abyssidrilus is commonly defined as a monophyletic group of species sharing slender, often sharply single-pointed, somatic setae, a synapomorphy unique for the subfamily ( Erséus 1992). Although more or less seen in the subterranean freshwater forms A. cuspis n. comb. and A. subterraneus n. comb., this character is not present in A. stephaniae Martin , n. gen., n. sp. in spite of its great similarity and assumed phylogenetic closeness to those species. Aberrantidrilus stephaniae Martin , n. gen., n. sp. has all somatic setae bifid, with teeth of similar length (see description below), which would render questionable the synapomorphy proposed for Abyssidrilus if the species were kept in the latter genus.
The location of spermathecal pores in XII is an exceptional condition in tubificids, so far only seen in Aberrantidrilus Martin , n. gen. species and Mexidrilus postspermathecus Erséus, 1980 . If only for the rarity of this condition, it is tantalizing to consider the latter species as the closest relative to this assemblage of thalassoid stygobionts. Mexidrilus postspermathecus is a subtidal oligochaete, known from the West coast of Norway ( Erséus 1980), with a depth distribution in accordance with the hypothesis usually put forward to explain the origin of thalassoid lineages, namely an evolution from marine ancestors by stranding following the regression of marine embayments ( Notenboom 1991; Holsinger 1993; Boutin & Coineau 2005). Assuming a derivation from the Abyssidrilus clade, the closest relative to the thalassoid species would be a deep-sea species, Abyssidrilus stilus , so far only found at about 4900 m depth in the Indian Ocean. This implies an invasion of the subterranean domain via an unknown common ancestor that lived at moderate depths ( Erséus 1992). Although sharing a unisetal penial “bundles” condition with Aberrantidrilus Martin , n. gen., Abyssidrilus stilus differs from the latter in having spermathecae in X, with spermathecal pores dorsal to the lateral line, and penial setae directed posteriorly.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Naidinae |