Epimeria schiaparelli, Maas, Anne-Nina Lörz Elizabeth W., Linse, Katrin & Fenwick, Graham D., 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.175450 |
|
DOI |
https://doi.org/10.5281/zenodo.5661106 |
|
persistent identifier |
https://treatment.plazi.org/id/CB3F8F46-FFAD-FF80-FFD2-FF74FB47FBD1 |
|
treatment provided by |
Plazi |
|
scientific name |
Epimeria schiaparelli |
| status |
sp. nov. |
Epimeria schiaparelli View in CoL sp. nov. ( Figs 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Material examined (all western Ross Sea, Antarctica)
Holotype: ovig.? (29.9 mm,), NIWA 18174, TAN0402/195, epibenthic sled, 71° 37.32–37.14' S, 170° 55.38–55.54' E, 28 February 2004, 244 m.
Paratypes: Each registration number refers to a single specimen unless otherwise stated; NIWA 18175, adult?, 21.6 mm, NIWA 18187, seven specimens, TAN0402/195, epibenthic sled, 71° 37.32–37.14'S, 170° 55.38– 55.54 E, 28 February 2004, 244 m; NIWA 18181, NIWA 18183, NIWA 18193, NIWA 18198, TAN0402/22, epibenthic sled, 71° 48.06'–47.91'S, 170° 56.48'57.56'E, 0 9 February 2004, 151 m; NIWA 18184, TAN0402/25, epibenthic sled, 71° 47.92–47.78'S, 170° 55.9756.95'E, 0 9 February 2004, 127 m; NIWA 18202, TAN0402/33, epibenthic sled, 71° 45.28–45.35'S, 171° 25.02– 23.94'E, 10 February 2004, 282 m; NIWA 18176, ovigerous, NIWA 18178, NIWA 18190, NIWA 18191, ZMB 27576–78, TAN0402/39, epibenthic sled, 71° 45.30–45.53'S, 171° 8.55–9.16'E, 10 February 2004, 251 m; NIWA 18180, ZMB 27576– 78 (former NIWA 18196), TAN0402/112, epibenthic sled, 71° 17.61–17.77'S, 170° 34.60–35.45'E, 18 February 2004, 346 m; NIWA 18194, TAN0402/116, epibenthic sled, 71° 17.93–18.21'S, 170° 32.43–33.02'E, 18 February 2004, 312 m; NIWA 18182, TAN0402/33, epibenthic sled, 71° 45.28–45.35'S, 171° 25.02–23.94'E, 10 February 2004, 282 m; NIWA 18188, TAN0402/189, van Veen Grab, 71°34.49'S, 170°52.24'E, 27 February 2004, 231 m; NIWA 18177, NIWA 18195, NIWA 18197, NIWA 18199, TAN0402/140, epibenthic sled, 72° 0.81–1.08'S, 170° 46.47–45.97' E, 26 February 2004, 231 m; NIWA 18192, TAN0402/190, Epibenthic Sled, 71° 34.75–34.97'S, 170° 52.37–52.43'E, 27 February 2004, 230 m; NIWA 18187, NMNZ Cr. 10024 (former NIWA 18201, 3 specimens), TAN0402/195, epibenthic sled, 71° 37.32–37.14' S, 170° 55.38–55.54'E, 28 February 2004, 244 m; NIWA 18179, TAN0402/197, epibenthic sled, 71° 37.24–37.11'S, 170° 51.99– 52.73'E, 28 February 2004, 198 m; NIWA 18185, TAN0402/198, Van Veen grab, 71°37.04'S, 170°53.61'E, 28 February 2004, 222 m.
Etymology
The species is dedicated to Dr Stefano Schiaparelli , who kindly shared his enthusiasm and knowledge on board during the BioRoss Antarctic expedition.
Description
Head higher than long, anterior cephalic margin sinuous, lateral cephalic lobe acutely produced; rostrum ( Fig. 1 View FIGURE 1 A, B) c. 1.5 x head length, reaching proximal part of antenna 1 peduncle article 2; eye oval, 0.5 x head height, set back from anterior cephalic lobe margin. Pereonite 1 subequal in length to head (excluding rostrum), pereonite 2 c. 0.5 x length of 1, pereonites 1 and 2 lacking middorsal or dorsolateral processes; pereonite 3 c. 1.3 x longer than 1, posterior margin with weak tooth appearing as thickening in lateral aspect, with weak dorsolateral process; pereonite 4 with blunt middorsal process overhanging pereonite 5, blunt dorsolateral carina weakly developed; pereonites 5–7 and pleonites 1–3 with large, acute middorsal teeth curved posteriorly to overhang following somite and distinct, dorsolateral processes, obtuse on 5–6, acute on 7 and pleonites 1–3. Epimeron 1 ( Fig. 6 View FIGURE 6 A) narrow, anteroventral angle rounded, with acute posterodistal tooth, posterior margin concave distally; epimera 2–3 similar to 1, transverse ventral margins increasingly longer, posterior excavations shallower, posterodistal cusps increasingly produced. Urosomite 1 ( Fig. 1 View FIGURE 1 A) with acute cone middorsally, lacking dorsolateral processes; urosomite 2 shortest; urosomites 2 and 3 lacking middorsal processes, with acute dorsolateral carinae at posterior margin.
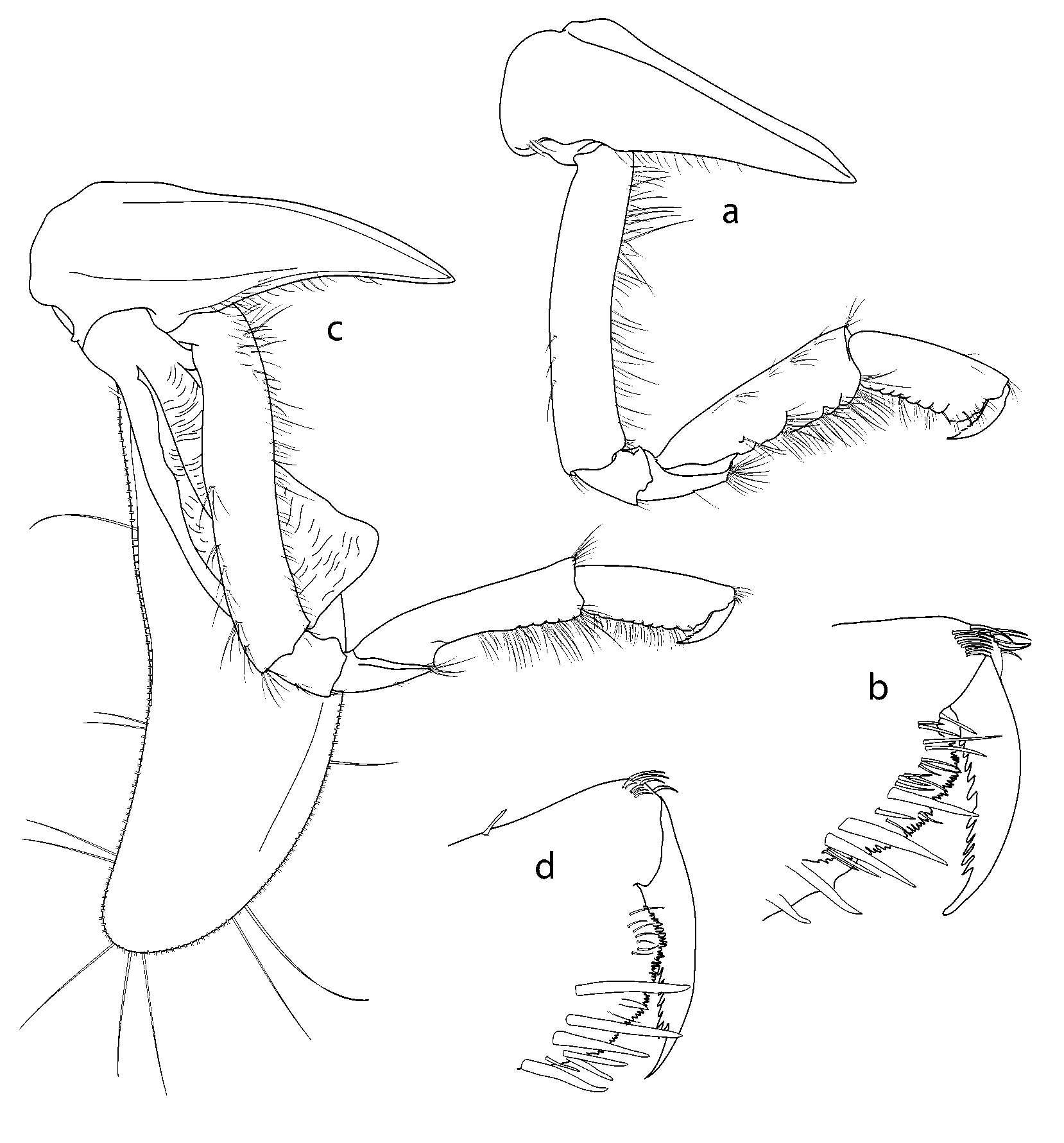
Antenna 1 ( Fig. 3 View FIGURE 3 A, B): peduncle article 1 with many plumose setae distal margin with 2 short and 1 long processes; article 2 with 2 long acute processes distally, length (including processes) slightly shorter than article 1; article 3 shortest; accessory flagellum scalelike; primary flagellum of 35 articles. Antenna 2 ( Fig. 3 View FIGURE 3 C) (longer than A1; article 2 with 2 large acute distal cusps; peduncle article 3 with short obtuse distal cusps; articles 4 and 5 lacking distal processes, lengths subequal; flagellum with 75 articles.
Mandible ( Fig. 2 View FIGURE 2 C): incisor and lacinia mobilis strongly dentate; molar produced and triturative; palp article 3 densely setose medially, with long stout SS distally. Lower lip ( Fig. 2 View FIGURE 2 E) (hypopharynx) with wide lobes and groups of setae on distomedial angles, hypopharyngeal gap narrow. Maxilla 1 ( Fig. 2 View FIGURE 2 B) medial plate subtriangular, obliquely convex inner margin with 11 stout, plumose SS; lateral plate distal margin oblique, with 11 medially lobate RS; palp strongly exceeding outer plate; palp article 1 short, article 2 slightly curved medially with stout SS distomedially, stout RS distally. Maxilla 2 ( Fig. 2 View FIGURE 2 A) with long, distally crenulate setae distally on lateral and medial plates. Maxilliped ( Fig. 2 View FIGURE 2 D) lateral plate broadly rounded distally, reaching midpoint of carpus, medial plate with nodular RS and a row of long plumose SS on medial, anterior face; palp medial margin strongly setose; merus distally expanded; dactyl with serrate medial margin.
Gnathopod 1 ( Fig. 4 View FIGURE 4 A, B): coxa 1 long and slender, anterior margin straight, broadly rounded anterodistally to form acute posterodistal corner, posterior margin straight; basis linear, slender, both margins with numerous fine SS; merus slightly longer than ischium, anterior margin very short, distal margin oblique, posterodistal angle acute, setose; carpus slightly expanded distally, distal margin transverse, anterodistal angle with SS, distal half of posterior margin with long SS; propodus subrectangular, 0.6 x carpus length, anterior margin naked except for distal fringe of short SS, palm finely crenulate, slightly oblique, with cluster of RS defining rounded distal margin, posterior margin with numerous long SS; dactylus slender, slightly curved, posterior margin strongly serrate. Gnathopod 2 ( Fig. 4 View FIGURE 4 C, D) slightly longer than gnathopod 1; coxa 2 similar in shape to coxa 1, tapering distally, posterior margin slightly concave; basis linear, extending 50% below coxa; merus short, 1.3 x ischium length, anterior margin very short, distal margin obliquely articulating with carpus, with group of 4 or 5 SS posterodistally; carpus curved proximally, widened distally to 0.3 x length, 4.5 x merus length, anterior margin naked except for transverse row of SS distally, posterior margin with numerous stout SS distally; propodus 0.6 x carpus length, 0.5 x as wide as long, palm almost transverse, rounded, finely crenulated, lined with numerous submarginal RS; dactylus large, slightly exceeding palm, posterior margin serrate. Pereopod 3 ( Fig. 5 View FIGURE 5 A): coxa wider and slightly longer than coxa 2, posterior margin strongly concave; basis linear, extending just further than coxa, anterior margin finely setulose, posterior margin with 7 groups of SS; merus slightly expanded distally, anterodistal angle weakly produced into narrow flange overhanging distal c. 0.2 of carpus; carpus c. 0.5 x merus length, slightly widened distally, anterior margin naked, posterior margin with 6 pairs of RS; propodus 1.3 x carpus length, naked anteriorly, posterior margin with 8– 10 pairs of RS; dactylus stout, curved, 0.5 x propodus length. Pereopod 4 ( Fig. 5 View FIGURE 5 B): coxa slightly longer than 3, 1.5 x longer than wide, anterior margin strongly convex, produced into stout, acute posterodistal cusp directed posterodistally, posterior margin divided at mid point by subacute cusp into two concave sections; basis to dactylus as for pereopod 3. Pereopod 5 ( Fig. 5 View FIGURE 5 D): coxa subrectangular, wider than long, posterodistal corner produced as slender cusp c. 0.5 x length of rest of coxa; posterior margin weakly concave; basis scarcely covered by coxa, expanded into irregular posterior flange with proximal rounded lobe and acute triangular posterodistal tooth; merus constricted proximally, posterodistally produced to overhang c. 0.2 carpus; carpus slightly widened distally, 0.8 x merus length; propodus sublinear, subequal in length to merus, posterior margin with 8 pairs of RS; dactylus curved, stout, c. 0.5 x propodus length. Pereopod 6 ( Fig. 5 View FIGURE 5 E): coxa anterior half hidden by coxa 5, anterior margin weakly concave, posterodistal corner produced into short, posterolateral tooth, posterior margin broadly rounded; basis to dactylus as in pereopod 5. Pereopod 7 ( Fig. 5 View FIGURE 5 C): coxa subrectangular, 1.1 x wider than long, slightly narrower distally; basis expanded posteriorly, margin sinuous, broadly rounded proximally, distal concavity forming acute cusp at posterodistal angle; ischium to dactylus as in pereopods 5 and 6.
Uropods extending equally, rami apices naked. Uropod 1 ( Fig. 6 View FIGURE 6 C): peduncle subequal in length to inner ramus, medial margin with few RS proximally and 1 distally, distal 0.6 of lateral margin with close row of short RS; inner ramus lateral margin with spaced row of short RS, medial margin with sparse RS; outer ramus marginally shorter than inner, marginal setation as in inner ramus. Uropod 2 ( Fig. 6 View FIGURE 6 D): peduncle naked except for 1 or 2 short RS distally on each margin; inner ramus length 1.3 x outer ramus, medial margin with sparse RS, distal lateral margin with close RS; outer ramus 1.2 x peduncle length, both margins with close rows of short RS over mid 0.5 of length. Uropod 3 ( Fig. 6 View FIGURE 6 E): peduncle short, c. 0.5 x length of inner ramus, produced into acute process extending c. 0.15 alongside inner ramus; outer ramus over 0.6 of its length, medial margin with sparse row of short RS along full length, inner margin with sparse short RS along distal 0.5 of length; outer ramus 0.8 x length of inner, almost twice as long as peduncle, lateral margin with dense row of RS over proximal 0.6 of length, medial margin with RS confined to distal 0.3 of length. Telson ( Fig. 6 View FIGURE 6 B) weakly tapering to c. 0.8 of basal width proximally, 1.2 x longer than wide, vshaped emargination 0.3 x length, lobes triangular, subacute, narrowly rounded apically.
Remarks
The new species, Epimeria schiaparelli sp. nov., superficially resembles Epimeria similis Chevreux, 1912 and E. macrodonta Walker, 1906 in the dorsal armature of pereonites 3–7 and pleonites 1–3. Epimeria schiaparelli , however, lacks carinae on pereonites 1 and 2, has a relatively short flexed rostrum and pereonite 2 is short relative to pereonite 1. The main differences between species of this complex are summarized in Table 1 View TABLE 1 .
Characters E. schiaparelli E. similis E. macrodonta Rostrum: head length 1:1 1:1 2:1 Morphological variation
The large dorsal pereonite and pleonite spines are so characteristic of species of Epimeria vary in size and shape in E. schiaparelli . The rostrum varies in relative length (mean rostrum: head ratio is 1:1.33 (n = 18)). In comparison, this ratio is greater in E. macrodonta (> 2:1) and up to at least 3:1, as in the newly designated lectotype.
The lateral spine on article 2 of antenna 1 always exceeds the length of the unproduced article (mean ratio spine: article is 5.9 (n = 19)) in E. schiaparelli . In E. macrodonta the lateral spine of article 2 of the first antenna is always longer than article 2. In E. similis , however, the lateral spine of antenna 1 article 2 is always shorter than the unproduced article.
Slight variation is present in development of the middorsal and dorsolateral processes on pereonite 3. In none of the specimens examined did the size of the pereonite 3 processes approach the equivalent processes on pereonite 4. A few specimens had slightly (c. 1.3 x) enlarged cusps on urosomites 2 and 3.
Similar variation is present in the development of the posteroproximal cusp on coxa 4 of E. schiaparelli . In some specimens, this cusp was represented by a small irregularity on the concave posterior margin. Usually, however, this cusp was strong and acute, with its length averaging 80% of the width of coxa 4 (n = 19).
Molecular results
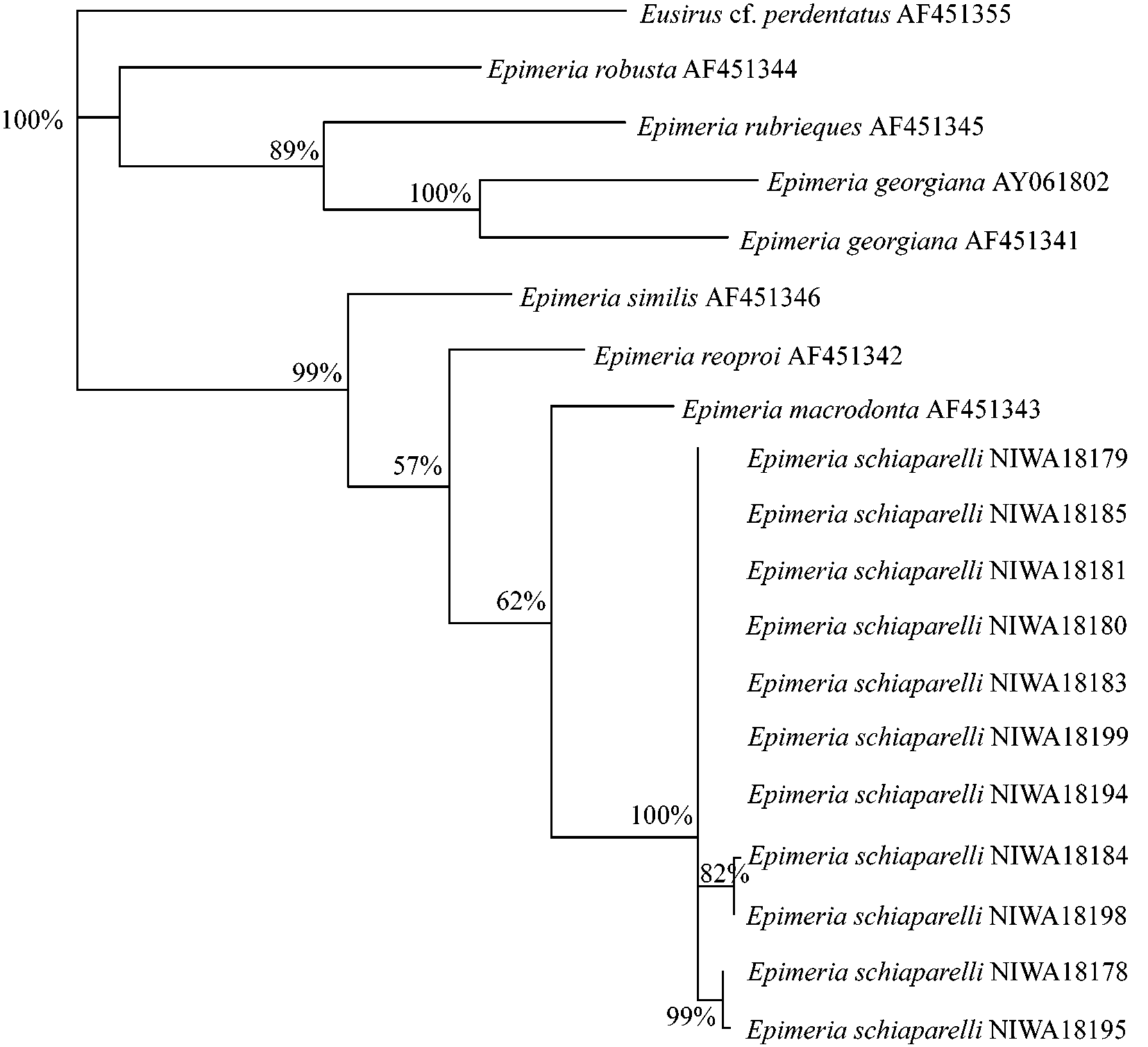
Analysis of the data recovered 12 most parsimonious trees (TL = 457, CI = 0.702, HI = 30298, RI = 0.717, RC = 0.503). The strict consensus and bootstrap values greater than 50% are shown in Fig. 8 View FIGURE 8 . Analysis of the partial COI gene showed a 0–2.19% sequence divergence within the E. schiaparelli specimens ( Table 2, Fig. 8 View FIGURE 8 ) and that these specimens form a distinct clade within Epimeria . This divergence within E. schiaparelli is much less than this group’s divergence from E. macrodonta (8.93–8.38%), the most closely related species. Divergences between other species were much larger (e.g., 12.02% divergence for E. similis and E. macrodonta ( Table 2, Fig. 8 View FIGURE 8 )) further supporting the conspecificity of all specimens identified as E. schiaparelli , despite conspicuous variation in some morphological characters.
Accession E. reoproi A B C D E F G H I J K L number
A— E. macrodonta AF451343 View Materials 10.75
B— E. similis AF451346 View Materials 11.66 12.02
C—E. schiaparelli AM176763 View Materials 11.11 8.93 12.93
(NIWA18181)
D—E. schiaparelli AM176764 View Materials 11.48 8.56 13.12 1.64
(NIWA18184)
E—E. schiaparelli AM398942 View Materials 11.11 8.38 12.57 0.55 1.09
(NIWA18180)
F—E. schiaparelli AM176765 View Materials 11.29 8.74 13.66 2.19 1.64 1.64
(NIWA18178)
G—E. schiaparelli AM176767 View Materials 11.48 8.56 13.12 1.28 1.09 0.73 1.64
(NIWA18179)
H—E. schiaparelli AM176768 View Materials 11.32 8.40 12.78 0.73 0.91 0.18 1.46 0.55
(NIWA18185)
I—E. schiaparelli AM176769 View Materials 11.14 8.40 12.60 0.55 1.09 0.00 1.64 0.73 0.18
(NIWA18183)
Validity of Epimeria similis and E. macrodonta
The identities of Epimeria macrodonta and E. similis have long been confused. This confusion started with Walker’s (1906) very short original description of E. macrodonta . Walker’s (1907) later, more detailed description and illustrations of this species referred to four specimens from two collections from the Antarctic Peninsula that constitute the syntype series. Examination of the syntypes, now held at the Natural History Museum, London, revealed one dissected specimen with most mouthparts and coxae missing. Further, the largest of the four specimens (Natural History Museum, London, labelled “No 13”) differs appreciably from the Walker’s (1907) description of E. macrodonta . In particular, it lacks lateral teeth on pereonite 1, pereonite 1 is 1.5 x pereonite 2 in length (pereonite 2:1 is 1.2 x pereonite 1 in the illustrated specimen) and the carinae on pereonites 3–7 and urosomites 1–2 are broader and shorter than those of the illustrated specimen. The largest specimen is not the same species as illustrated by Walker, and the type series is a composite. We draw the conclusion that the type series comprises more than one species and therefore a lectotype designation is necessary to fix the identity of the species. The paralectotypes, including the ‘large’ specimen, are identified as Epimeria cf. similis , for which further genetic characterisation will hopefully give final clarification. Therefore, another specimen (Natural History Museum, London, labelled “J107”) which closely resembles that described and illustrated by Walker (1907) is here designated the lectotype of E. macrodonta .
In erecting E. similis, Chevreux (1912) noted that it differed from E. macrodonta Walker, 1906 in several characters, including the presence of a dorsal carina on pereonite 2. The validity of this separation was supported by Schellenberg (1926) who added that E. similis possessed dorsolateral teeth on all pereonites. Subsequently, Barnard (1930) synonymised the two species, regarding many of Walker’s (1907) and Chevreux’s (1912) reported differences between the two as morphological variation. Despite making the synonymy, Barnard (1932) continued to identify two forms within Discovery expedition material: E. macrodonta forma macrodonta (one juvenile specimen from the Palmer Archipelago) and E. macrodonta forma similis (80 specimens from South Shetlands and the Palmer Archipelago).
Watling & Holman (1981) accepted Barnard’s (1930) synonymy, identifying their northern Antarctic Peninsula material as E. macrodonta forma similis , and regarding as aberrant the one specimen (of 14) that bore a midventral tooth distally on antenna 1 peduncle article 2, and lacked a carina on pereonite 1.
Andres (1985) checked Barnard’s (1932) Discovery material and subsequently regarded E. similis as a valid species. According to Andres (1985), E. similis is the only species of Epimeria with lateral teeth on all pereonites and pleonites, and lacking a dorsal carina on pereonite 2, whereas pereonites 1–2 are smooth in E. macrodonta . Andres (1985) may have not checked the type material of E. macrodonta , because the largest syntype does bear lateral teeth on pereonites 1–2 (contrasting with Walker’s (1907) description and illustrations of E. macrodonta ). The present investigation confirms the validity of E. similis based on type material in the Muséum national Histoire naturelle, Paris, genetic evidence, and from a better understanding of the intraspecific morphological variation within the closely related E. schiaparelli .
Distribution of Epimeria macrodonta complex species
The distributions of the three species comprising the Epimeria macrodonta complex can now be clarified. Epimeria macrodonta appears to be limited to the Weddell Sea and the Antarctic Peninsula at 0–900 m depth, and has not been found in the Ross Sea ( Andres 1985; present study). Epimeria similis is distributed throughout the eastern Antarctic and the Scotia area, inhabiting 165–420 m depth ( Andres 1985). In comparison, E. schiaparelli has been found only in the Ross Sea, at 130–350 m depth.
Variation and radiation of Epimeria cf. similis
In addition to E. schiaparelli , the BioRoss Expedition 2004 collected more than 40 specimens of an Epimeria species that closely resembles E. similis . These specimens closely resemble E. similis morphologically, but had only a single acute process on coxa 4. These specimens also differed from E. similis in that the distal spines on antenna 1 peduncle articles 1–2 were shorter, pereonite 2 was longer and the acute lateral process on coxa 5 was shorter than in E. similis . Since subtle morphological distinctions have proven to be reliable in the distinction of Epimeria species, we have good evidence that we have another new species within the Epimeria similis complex. It is presently under study and the further genetic studies will reveal its phylogenetic position.
Molecular data
In a morphological analysis of Antarctic Epimeria, Lörz & Brandt (2004) noted that some species, such as E. similis and E. robusta , are highly variable morphologically. They suggested that recent speciation of the Epimeriidae had occurred in the Southern Ocean. Molecular data on the phylogeny of Antarctic Epimeria species ( Lörz & Held 2004) supports the theory of recent speciation when Antarctica cooled, and after the Drake Passage formed.
The molecular data presented here clearly supports E. schiaparelli as a new species, with the nearest congeners, E. macrodonta , E. reoproi and E. similis , all showing greater than 8% sequence divergence. This level of sequence divergence is similar to that previously documented between species of Epimeria (see Lörz & Held 2004). In addition, Väinölä et al. (2001) observed 4.8–12.5% COI gene sequence divergences between species of the amphipod genus Gammaracanthus Bate, 1862 . These interspecific data are consistent with our findings, which show a minimum interspecific divergence of 8.38% between Epimeria species. The intraspecific variation within the 11 specimens of E. schiaparelli sequenced was 0–2.19% sequence divergence, corroborating the validity of E. schiaparelli despite the small morphological differences observed.
Colouration
Two colour patterns were recorded from freshly captured specimens of E. schiaparelli ( Figs 7 View FIGURE 7 A, B). The most common form was a pattern of irregular orange patches ( Fig. 7 View FIGURE 7 A), but five specimens (NIWA 18177– 18180) had distinctive red–orange bands when alive ( Fig. 7 View FIGURE 7 B). Large and small specimens of both males and females had each colour pattern, so colouration was not size or sex related. The colour pattern was also independent of locality or depth of occurrence. Further, molecular data using the COI revealed that the different colour morphotypes do not form a separate clade and that the sequence differences between the two coloured forms (NIWA18180, NIWA18178 and NIWA18181) and the other morphotype (all other sequences) is the same as the interspecific divergence (0–2.19%). The sequence divergences within the colour morphotypes is 0.55–2.19% and the divergence within the non coloured morphotype is 0.0–1.83.
TABLE 1. Summary of morphological differences between Epimeria schiaparelli n. sp., Epimeria similis Chevreux, 1912 and E. macrodonta Walker, 1907.
| Antenna 1 article 2 lateral spine reaches article 3 | Yes | No Yes |
|---|---|---|
| Pereonite 1 middorsal process | None | None Thickened posterior margin |
| Pereonite 2: l middorsal length | 1:2 | 1:1 1:2 |
| Pereonite 2 middorsal process | None | Thickened posterior None margin |
| Pereonite 2 posterolateral process | None | Weak None |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |