Mata ruffordii, Sarkar & Mahapatra & Mohapatra & Nair & Kunte, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4908.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E342A22E-045A-4364-A4C8-839C00FEB5F6 |
|
DOI |
https://doi.org/10.5281/zenodo.4442941 |
|
persistent identifier |
https://treatment.plazi.org/id/CA083717-B960-D008-7DF0-E4E6FEFDF9F5 |
|
treatment provided by |
Plazi |
|
scientific name |
Mata ruffordii |
| status |
sp. nov. |
2. Mata ruffordii View in CoL sp. nov.
(Map 1; Figures 6,7,8,9,14)
2.a. Type Material details: Holotype: Collection Voucher Code is VS-AA436 and Specimen Voucher Code of the holotype is NCBS-BI001 .Male.The type locality of this species is Balpakhram National Park ( BNP), ( 25°14'46.77"N, 90°51'41.83"E) South Garo Hills , Meghalaya (Map-1). The specimen was collected on 13 th September , 2017 by Vivek Sarkar. The specimen was preserved in ethanol after collection. Two legs and a chunk of thoracic tissue was preserved in absolute ethanol and the specimen was pinned and dried later in December , 2019. It is deposited in the Research Collections Facility at NCBS, Bengaluru (=Bangalore), India GoogleMaps . Paratype: Collection Voucher Code is VS-AA436 and Specimen Voucher Code of the paratype is NCBS-BI002 . The locality is forested area of Laitrengew ( 25°19’53.95”N, 91°43’58.74”E), East Khasi Hills , Meghalaya. The specimen was collected on 15 th September , 2017 by Vivek Sarkar. The specimen has been preserved in the same manner and pinned and dried later in December , 2019. It is deposited in the Research Collections Facility at NCBS, Bengaluru (=Bangalore), India GoogleMaps .
2.b. Diagnosis: This species appears as a combination of characters from the two previously known species of Mata . Similar to M. rama but unlike the prominent infuscation only on the radial and radiomedial crossvein of the forewing, this species has conspicuous infuscation on the radial and radiomedial crossvein; first and second cubitus anterior vein; all distal median veins; mediocubital crossvein and median crossvein (Fig-8A&B), similar to that of the M. kama ( Distant 1881; 1906). But unlike the completely green with very thin black margined male opercula of M. kama (Fig-1B), this species has greenish brown male opercula with broad dark edges which is suffuse to some extent (Fig-8B) similar to M. rama but not as extensively suffused (Fig-2B). Timbal cover of this new species matches that of M. kama to some extent, having an anterior angular black spot which does not extend dorsally. But unlike M. kama , where the remaining timbal cover is green with overlaid white scales only at the posterior part of the lateral side (Fig-1C), this species has a predominantly white timbal cover which is overlaid with fine white scale (Fig-8C&D). In both of these species traces of these overlaid white scales clearly extend dorsally and laterally on the third tergite.
2.c. Etymology: This species was first discovered under a tenure of a project that was supported by the Rufford Foundation ( UK), part of the Rufford Small Grant programme. The Rufford Foundation has been supporting various conservation related work of many young researchers in India and many other countries for more than a decade. The species is named as ‘ ruffordii ’ to honour the support of the Rufford Foundation in conservation of nature and wildlife, starting from the smallest invertebrates to majestic mammals and their habitat.
2.d. General Measurements:
2.e. Type series: Holotype: “ INDIA / S. G. Hills Dist. , Meghalaya / BNP / Vivek S coll./ VS-AA436 / 13.ix.2017 / NCBS-BI001 ”, male ( NCBS). // Paratype: “ INDIA / E. K. Hills Dist. , Meghalaya / Laitrengew / Vivek S coll./ VS-AA437 / 15.ix.2017 / NCBS-BI002 ”, male ( NCBS) .
2.f. Description
2.f.1. Holotype
Head: Postclypeus greenish brown with prominent black median part and black transverse groove fading laterally. Eyes brown with tinge of green and ocelli pale sanguine, more pinkish. Epicranium green towards posterior end adjacent to pronotum and gradually turns brown anteriorly in live insects but the entire vertex turns pale brown in pinned specimens. Dark supra-antennal plate with a greenish brown spot at the top. Vertex with black patches adjacent to eyes, broadens anteriorly and extends to the posterior part of supra-antennal plate. This dorsolateral black patch encircles a triangular pale brown patch between eye and supra-antennal plate. Pedicels brown, antennal flagellum black.Area around the ocelli black, mark extends posteriorly, adjacent to pronotum and anteriorly to frons but does not enter into the frons, adjacent to postclypeus. Lorum black. Anteclypeus brown with inverted black ‘T’ mark and bottom half of the anteclypeus with black outer margin as an extension of the horizontal arms of this ‘T’ mark. Rostrum brown with less than one fifth of its length black at the tip.
Thorax: The base colour of the pronotum green, turning brown gradually at the centre in live specimens and entire uniformly brown thorax in pinned specimens. Pronotum with a mid-dorsal brown arrow-shaped marking pointing posteriorly. This median arrow-shaped marking surrounded by a broad black margin, appearing in the shape of a pawn chess piece with its broad base facing anteriorly, adjacent to head. Area between lateral fissure and paramedian fissure with irregular patches of black. Thin black border at the inner lateral part of pronotal collar. Lateral part of pronotal collar with broad black outer margin to the lateral angle where it broadens and curves internally, making the posterior part of lateral angle look distinctively greener. Posterior part of the pronotal collar green with thin black margin including the pronotal collar lateral angle. A thin black dorsolateral line at medial pronotal collar lateral angle crossing pronotal collar on both sides. Mesonotum greenish brown with green lateral side that looks like the extension of the green pronotal collar lateral angle in live specimens. Mesonotum with a mid-dorsal black arrow-shaped marking, pointing posteriorly. Parapsidal suture brown. Submedian sigilla with a fish hook or “J” shaped black spot adjacent to the parapsidal suture. Lateral sigilla with a black “Y” shaped mark, adjacent to the parapsidal suture. Black spot encircles black scutal depression, appearing as elongated, somewhat comma-shaped black dots at the base of the mesonotum. Scutellum plain green in live insects which turns uniform brown in pinned specimens. Metanotum beyond wing groove black with green patch before the black tip. Forewing with greenish brown amber tinge that darkens at the base. Infuscation at the tip of the transparent basal cell of forewing. Prominent infuscation on radiomedial crossvein, first and second cubitus anterior veins, all submarginal median veins, mediocubital crossvein and median crossvein of forewing. Costa of the forewing greenish brown to node and dark brown past the node. Basal veins such as arculus, cubitus anterior veins, cubitus posterior veins, median vein of the forewing greenish brown in live insects which gradually turn dark in distant veins. The greenish brown colour of basal veins of live insects turns pale brown in pinned specimens. Median vein prominently white at node, proximal to the confluence of radius anterior and radius posterior. Hindwing completely transparent with black to dark brown veins except the black base of cubitus anterior vein and first anal vein. The basal membrane of forewings and jugum of hindwings greyish black. Legs pale greenish brown with dark brown patches at the joints of trochanter, femur and tibia. Tarsi of foreleg and midleg entirely dark brown. Tarsi and tibial spurs including the tibial comb of hind leg pale greenish brown with dark brown pointed tip. Meracanthus black with pale brown outer edge.Male opercula short and greenish brown with broad dark edges which, to some extent, suffused posteriorly. Ventral thorax overlaid with fine white scales.
Abdomen: First tergite appears white in live specimens with overlaid fine white scales and brown with posterior black margin in pinned specimens. Second tergite greenish brown in the middle with thin green posterior border and black lateral border, adjacent to timbal cover. Timbal cover predominantly white, overlaid with fine white scales with anterior angular black spot which does extend dorsally. Traces of white scales clearly extend dorsally and laterally on the third tergite. Third to eighth tergites chestnut brown with darker posterior edge. Abdomen ventrally overlaid with pollinosity in live insects. First sternite dark brown, second to sixth sternites uniformly chestnut. Seventh sternite chestnut which gradually darkens posteriorly, adjacent to the eighth sternite. Eighth sternite dark brown with two ventrolateral pale brown oblong spots.
Male Genitalia: As shown in the Fig.14 C&D View FIGURE 14 . Pygofer pale brown which turns dark gradually at the protruded distal shoulder. Dark brown edge of the pygofer from the rudimentary upper lobe to the distal shoulder gradually turns darker. Prominent dorsal beak dark with brown hair like structures. Anal style and anal tube pale brown with overlaid hairy structures. Median lobe of uncus beige, broadened laterally and flat at the tip with a minutely protruding notch adjacent to the opening of the aedeagus ( Fig.14 C View FIGURE 14 ). Chestnut aedeagus tube-like with tapered end and slender white membranous gonopore which does not extend dorsally.
2.f.2. Paratype: Very similar to the holotype with darker and more conspicuous spots in the head and thorax due to hyper pigmentation.
2.g. DISTRIBUTION: In addition to the localities of the type series, the species has been recorded in the Nokrek Peak area of Nokrek National Park, West Garo Hills and the entire Cherrapunjee-Mawsynram plateau and its slopes, East Khasi Hills, especially the elevated forested parts and steep valleys (Map-1). The species is more common in parts of the Khasi Hills than Garo Hills, with the highest congregation recorded at the Nohkalikai Slope of Sohra (Cherrapunjee), East Khasi Hills. Other places where large congregations were also observed include the Wahkaba Valley of Sohra (Cherrapunjee) that opens towards Nongpreyang village, Khasi Hills; Ladmawphlang Valley which is the continuation of Mawkodok Valley, Khasi Hills; and the steep slopes of southern valley of Mawsinram, (on the way to Ranikore-Baghmara road) East Khasi Hills (Map-1). The species is desideratum for Jaintia Hills, especially the higher forested slopes and valleys of Saipong Reserve Forest.
2.h. BIONOMICS
2.h.1. Habitat type: This species is primarily recorded at the edges of well forested slopes, 1100 meters ASL and above. The species is occasionally seen in the edges of riparian forest of the plateau ( Fig.7D View FIGURE 7 ). It appears to prefer thick forest edges with Bamboo, species of Prunus L., Rhododendron L., and Castanopsis (D. Don) Spach.
2.h.2. Annual adult activity period: The activity of this cicada was only noted in 2017. The first individual was spotted during the second week of September in parts of Garo Hills and Khasi Hills. Peak activity was recorded during the first week of October. The last individual was recorded at the beginning of the third week of October, 2017.
2.h.3. Behaviour: Crepuscular species, active only at the dusk and does not call at dawn. Dendrophilous in nature, stays inactive the entire day except for jet spraying once in a while. Males stay at the thick canopies of the trees during the day time while females stay on the lower part of the same tree or nearby bushes. Males emit timbalizing signals right after the roosting calls of birds and continue until the beginning of Chiropteran activities, leaving a very narrow window for acoustic activity. This behaviour was studied from 11 th September 2017 to 10 th October 2017 both in Garo and Khasi Hills and it was found that male timbalization sessions last only for 40 minutes on average per day. Sub-gregarious in nature, males emit timbalizing calls rigorously from nearby trees, bushes and shrubs simultaneously, making it difficult to record a single call. They often keep on changing their perch position while calling. Individuals responds to pre-recorded calling songs. It was observed almost on a daily basis that after the sundown, towards the end of their calling session, bats hunt the cicadas, most likely by locating the cicadas by their calls. The bats swiftly approach the calling insect, grab it in their mouth and fly away. Once captured by the bat, timbalizing males makes a continuous buzzing noise, different from the usual advertising call, somewhat as a distress call which may be perceived as warning call for other males to terminate the chorus for the day. However, this needs to be supported with quantitative ecological experiments. In the evening these bats hunt boldly and do not get affected by human presence in close proximity to the calling cicadas. In one incident on 27 th September 2017, an individual of this cicada species was spotted calling from a lower perch of Prunus , L. tree behind St. John Bosco Boys Secondary High School at Maraikaphon of Sohra (Cherrapunjee), East Khasi Hills. While swinging the handheld net, an approaching bat, which was about to capture the same cicada, was caught in the net instead of the targeted insect. The bat was photographed and released immediately and later, it was identified as a Horseshoe bat, Rhinolophus sp. ( Fig.7D View FIGURE 7 ).
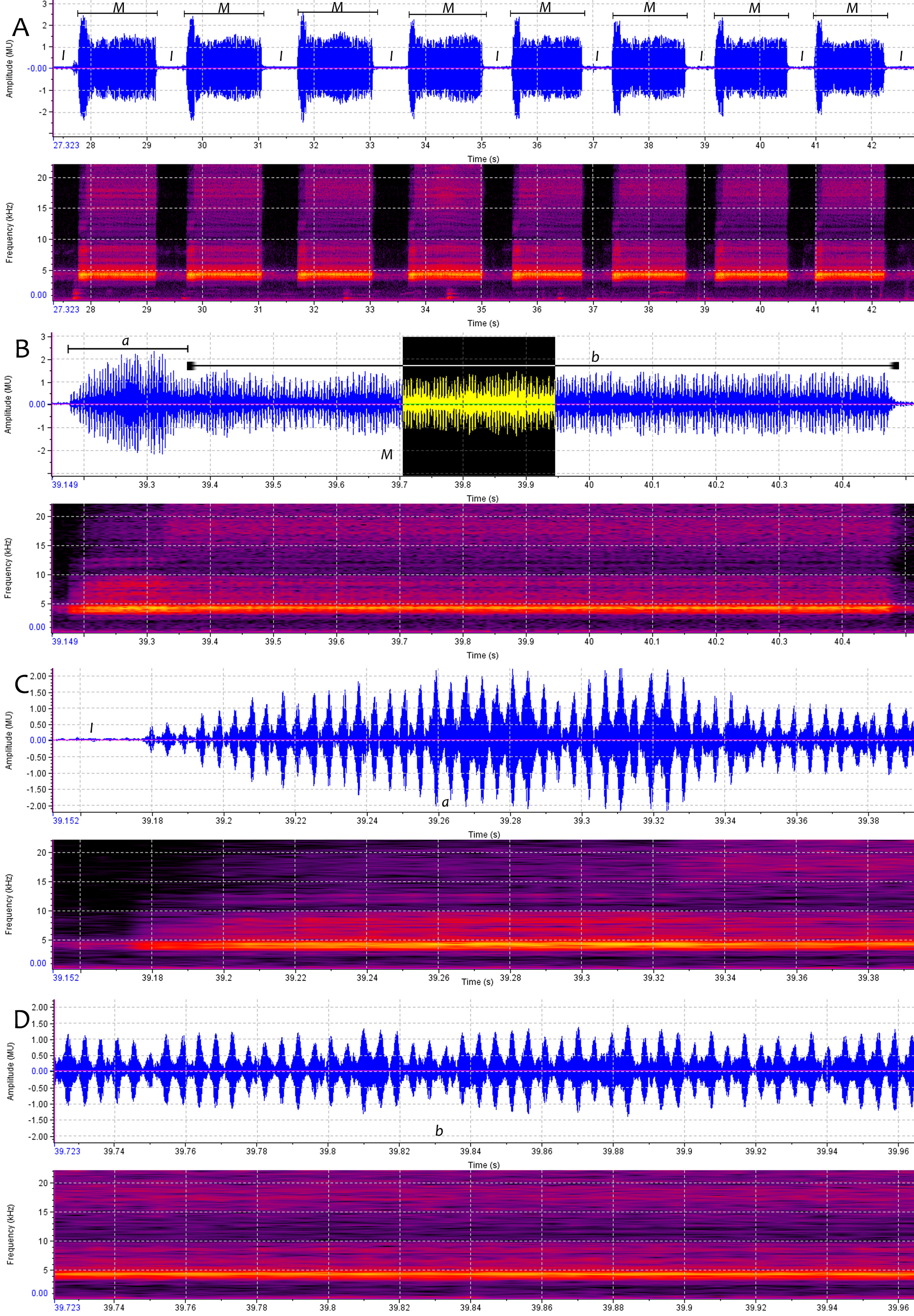
2.h.4. Acoustics: Male timbalization consists of short, near identical echemes M, repeated continuously for as long as 40 minutes. Fig.9A View FIGURE 9 is a temporal oscillogram and spectrogram illustrating about 15.5 seconds of the male timbalization, showing 8 of these repetitive echemes M. Each echeme M lasts for 1.373 seconds on average (range 1.284 to 1.432 seconds, n=22). The intervals I between the echemes M are.539 seconds long on average (range.448 to.606 seconds, n=23). The spectrograms appear to show a harmonic series at intervals of about 4 kHz. Fig.9B View FIGURE 9 illustrates an oscillogram and spectrogram of more than 1.4 seconds to show a complete echeme M consists of pulses produced at a rate of about 189/second, with the average first 0.159s (n=22) of each echeme, ‘ a ’, produced at a higher amplitude, almost twice as loud than the rest, ‘ b ’. Fig.9C View FIGURE 9 illustrates an oscillogram and spectrogram extending in an arbitrary space-time unit of 0.238 seconds to show the signal ultrastructure of the spindle-shaped, loud, initial ‘ a ’ of the echeme M and Fig.9D View FIGURE 9 illustrating an oscillogram and spectrogram of the inverted part of the Fig.9B View FIGURE 9 to show the signal ultrastructure of part of the prolonged posterior ‘ b ’ of the echeme M. The spectrogram corresponding to the oscillogram illustrates a wide frequency spread, from 3KHz to 20KHz and beyond. The sound energy is strongly concentrated at approximately 4.2 kHz.
2.i. Proposed Common Name: Rufford’s Spotted-back cicada
2.i.1. Justification: The justification of calling all the species classified in the genus Mata as ‘Spotted-back cicadas’ is explained in the common name of Mata lenonia sp. nov. This species is named ‘ ruffordii ’ to honour the support of the Rufford Foundation in conservation of nature and wildlife, the species is called Rufford’s Spottedback cicada.
| BNP |
Banff Park Museum |
| NCBS |
Yale University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.