Arbocuspis emanuelae, Rosso & Sciuto & Sanfilippo & Jones, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4282.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:049026D4-2D2E-4443-98ED-FD7B4320A976 |
|
DOI |
https://doi.org/10.5281/zenodo.6001249 |
|
persistent identifier |
https://treatment.plazi.org/id/C11C8789-FFAC-FF9C-FF6A-276FFB86FE3B |
|
treatment provided by |
Plazi |
|
scientific name |
Arbocuspis emanuelae |
| status |
sp. nov. |
Arbocuspis emanuelae n. sp.
( Figs 2–21 View FIGURES 2 – 4 View FIGURES 5 – 8 View FIGURES 9 – 13 View FIGURES 14 – 21 )
Material examined. Holotype: PMC.B 20 . 28.6.2008 a, the largest colony lobe encrusting the surface of a partly preserved Gastrochaenolithes in a broken sandstone pebble from sample BN 28, collected slightly South of Laem Pakarang, at 8°41.280’ N and 89°12.203’ E, in 14.7 metres, off southwestern Thailand, in the Andaman Sea, Recent. Paratypes: PMC.B 20 GoogleMaps . 28.6.2008 b, smaller colony lobes on the same broken sandstone pebble seemingly belonging to a different colony. PMC.B 20. 28.6.2008 c and PMC.B 20. 28.6.2008 d. Two further colony portions on a millimetre sized and centimetre sized broken sandstone fragments, respectively. All specimens from the same sample as the holotype.
Etymology. Named after Dr Emanuela Di Martino, whose invaluable studies are contributing to shed light on the origin of bryozoan biodiversity in tropical oceans.
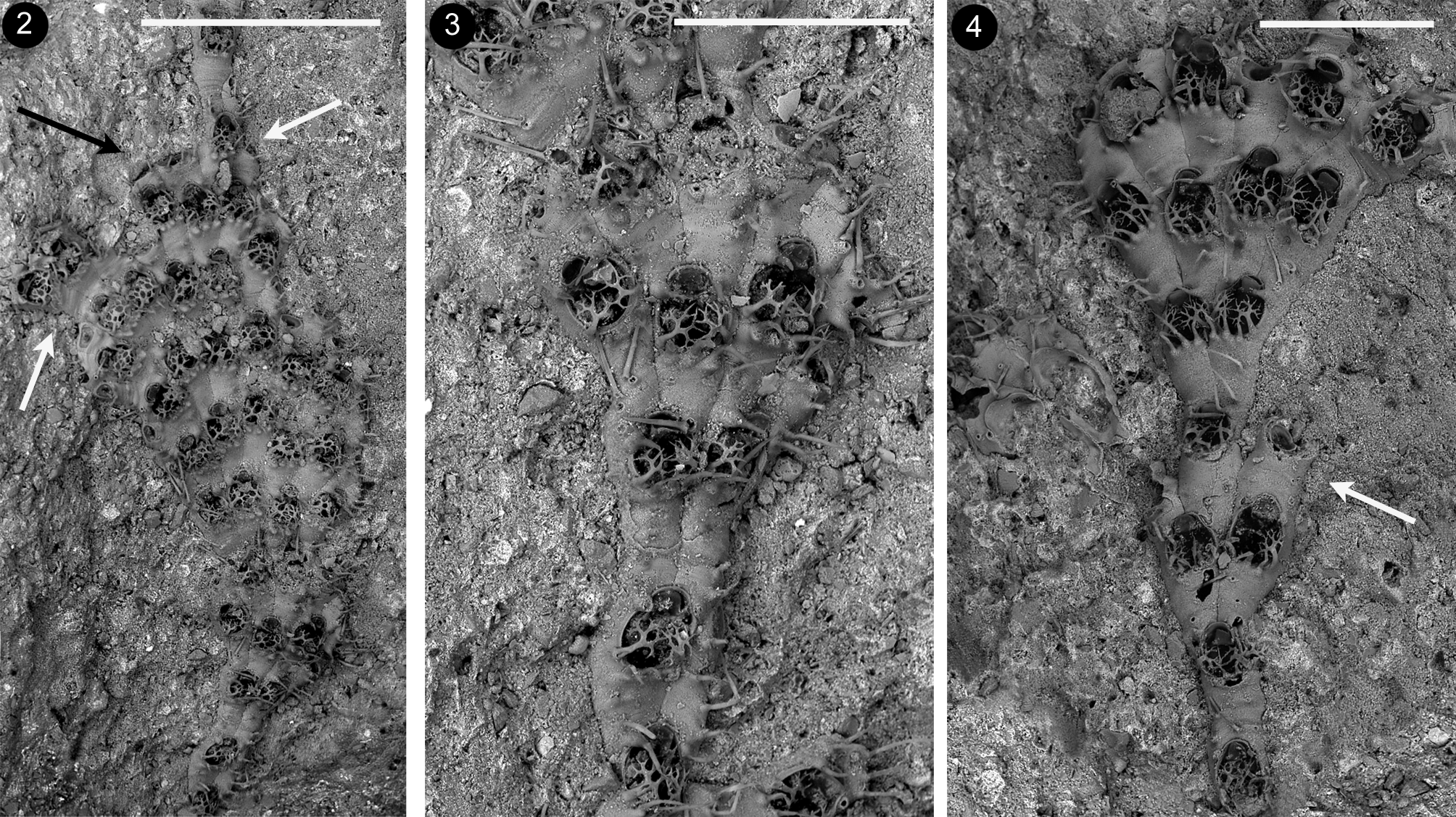
Description. Colony small-sized, encrusting, unilaminar, uni– to pauciserial, typically developing as a succession of either fan–shaped lobes or nearly parallel–sided ribbon–like lobes ( Figs 2–4 View FIGURES 2 – 4 ), which consist of transverse rows of flanked zooids. Lobes often rapidly increase in width at the beginning, because of the particular zooidal budding pattern involving: 1) the usually simultaneous budding of all zooids in a transversal row; 2) the formation of two new zooids distally to each parental one, except for lateral zooids usually budding a single distal zooid plus a kenozooid; 3) the absence of additional budding loci along the lateral walls. Two adjacent zooids in a tranversal row may occasionally produce a new zooid in between them besides two lateral ones ( Fig. 4 View FIGURES 2 – 4 ). In this case two adjacent zooidal buds fuse forming one cystid. Lobes include up to eight zooids in a transversal row. New fans/ribbons are formed: 1) along the sides of previous lobes, originating from the external distal bud of a lateral zooid diverging from the neighbouring zooid in the same transversal row ( Figs 9–10 View FIGURES 9 – 13 ), and 2) from ‘isolated’ zooids along the distal edge of previous fans ( Figs 2 View FIGURES 2 – 4 , 16 View FIGURES 14 – 21 ) which stop their growth. Halting of growth along the lobes’ sides and the growing edge can also occur through the development of kenozooids.
Autozooids slightly calcified, whitish and vitreous, elongate 0.411–0.482; 0.443± 0.025 mm long (n=12) and 0.164–0.276; 0.201± 0.037 mm wide (n=12) ( Figs 5–6 View FIGURES 5 – 8 ). Terminal oval to pyriform opesia narrowing distally, in correspondence of the operculum, and abruptly widening proximally being 0.179–0.223; 0.197± 0.013 mm long (n=12) and 0.145–0.174; 0.157± 0.010 mm wide (n=12). Zooidal proximal portion is an extensive gymnocyst narrowing proximally and ending into a pointed or bifurcating area(s) deeply wedged in between zooids of the previous row.
Gymnocyst extended proximally about half of zooidal length, 0.205–0.273; 0.247± 0.026 mm long, and only forming a narrow rim lateral to the opesia (ca. 0.020–0.030 mm wide, exceptionally more), narrower along the distal border. Gymnocystal surface smooth but occasionally slightly wrinkled by transversal growth marks. Cryptocyst as a narrow shelf on the inner margin of the gymnocyst. Peripheral rim of the opesia locally marked by a faint crenellation. Two large spines are placed close to the proximolateral rim of the opesium, symmetrically arching over the frontal membrane, branching dichotomously and fairly symmetrically four times, forming 16 minute pointed tips when fully developed, tips of these two spines nearly joining/overlapping in the middle ( Figs 5–6 View FIGURES 5 – 8 , 14–15 View FIGURES 14 – 21 ). Three to four unbranched median spines, up to 0.150–0.170 mm long, sharply pointed, more or less elongate, almost aligned along the proximal border of the opesium. Additional, usually smaller, spines of comparable shape occur on the proximal gymnocyst, consisting of a calcified basal portion continuous with the gymnocyst, and a chitinous pointed ending portion. One of such spines, usually the most developed and possessing a stout conical calcified portion, placed proximally, lateral to the orifice of a proximal zooid so that spines from the two zooids distal to an orifice give the impression of oral spines ( Fig. 6 View FIGURES 5 – 8 ).
Kenozooids either (i) smaller than autozooids, with a slender subtriangular to elongated trapezoidal morphology narrowing proximally, or (ii) equal size and shape as autozooids, nearly completely consisting of an extended gymnocyst marked by heavy transversal wrinkles often corresponding to minute bulges along the edges, bearing two–four scattered pointed spines, mostly on the proximal portion ( Figs 10 View FIGURES 9 – 13 , 14–16 View FIGURES 14 – 21 ). Opesium usually distal to subterminal, transversally oval to rounded, covered by a membrane. Closure plate (when present) faintly granular, centrally sinking, marked by nearly concentric calcification rings and ending in a central subcircular to oval fenestra. Triangular to rectangular elongated kenozooids are typically budded at distal–lateral corner(s) of zooids located along the lobes’ sides marking the end of longitudinal rows of zooids ( Figs 14–15 View FIGURES 14 – 21 ). Larger kenozooids are formed distally to some or all the zooids in a transversal row at the end of lobes ( Figs 2 View FIGURES 2 – 4 , black arrow; 16 bottom left). In damaged colonies small and roundish, to irregularly shaped kenozooids sometimes with long cauda–like projection and nearly central opesia occur ( Figs 17, 20 View FIGURES 14 – 21 ). More rare small swollen kenozooids are budded frontally, aligned along vertical walls of contiguous zooids. Multiporous septula are present in the laterodistal corners of transversal zooidal walls, and presumably along their lateral walls. Ancestrula was not observed.
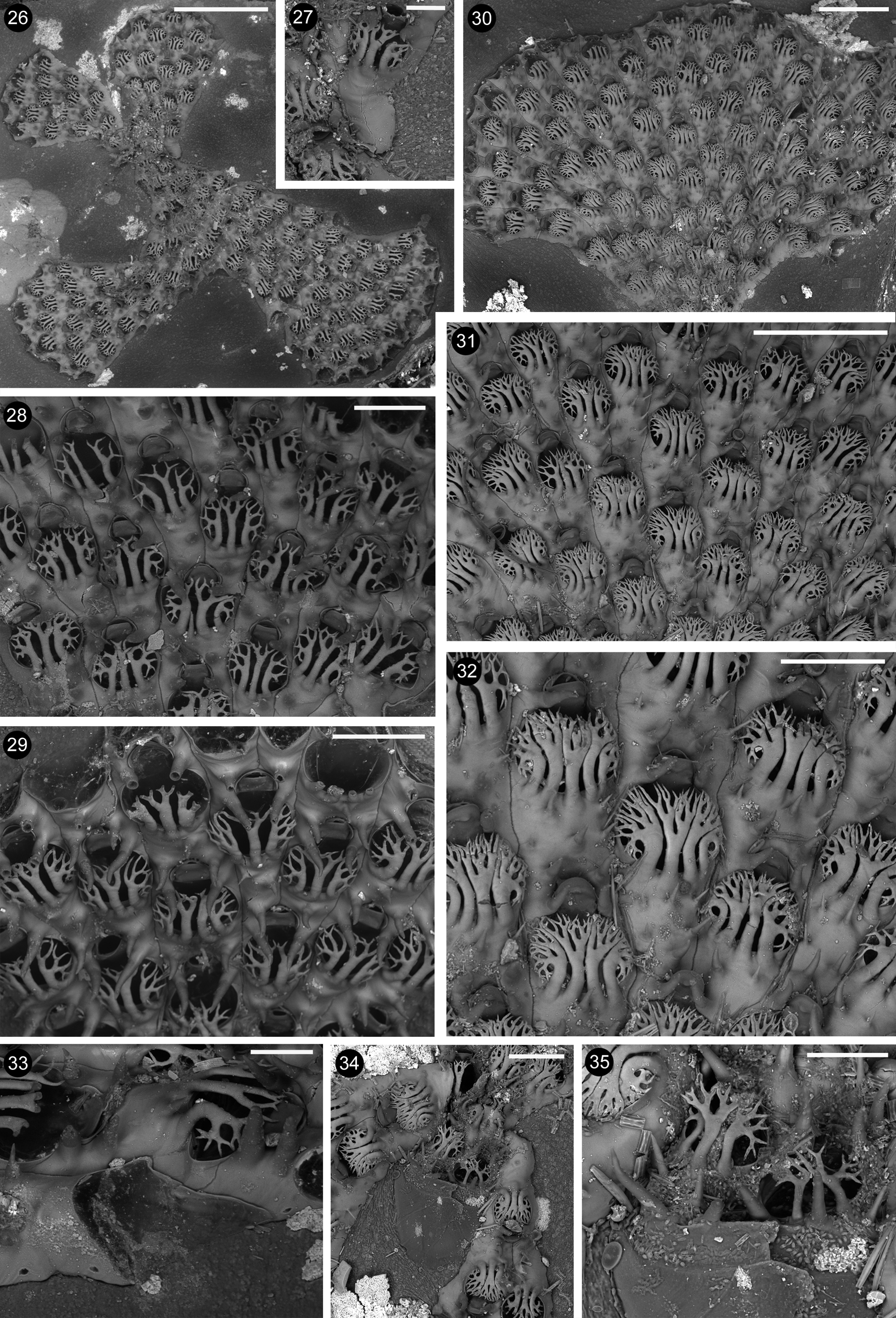
Remarks. Arbocuspis emanuelae n. sp. resembles Arbocuspis bicornis ( Hincks, 1881) in having two bifurcating spines arching on the opesium. Nevertheless, specimens from Thailand strongly differ from the type material of A. bicornis from Ceylon ( Figs 22–25 View FIGURES 22 – 25 ), and from the specimens of possibly the same species collected in Alagoas, northeastern Brazil, and figured by Vieira et al. (2016, their Figs 26-27 View FIGURES 26 – 35 ). Arbocuspis bicornis possesses a constant single conspicuous and stout central spine, raising from the very prominent proximal gymnocyst, and located in between the two lateral branched ones. Lateral spines are aligned with the central one in A. bicornis , whereas they are inserted in a slightly distal position in relation to the group of three-to-four central spines in A. emanuelae n. sp. This species also lacks an evident cryptocyst and only slight crenulations have been occasionally observed on the opesial rim. In contrast, a thin cryptocyst is present, with one/two rows of coarse granulations in the type material of A. bicornis ( Figs 24–25 View FIGURES 22 – 25 ), although it seems to be lacking in specimens from Brazil figured by Vieira et al. (2016, their Fig 27 View FIGURES 26 – 35 ). Further differences include the sizes of the opesia and, above all, of the zooids that are sensibly smaller in A. bicornis , which has opesia 0.157–0.179; 0.163± 0.004 mm long and 0.133–0.169; 0.152± 0.012 mm wide, and zooids 0.315–0.351; 0.329± 0.004 mm long and 0.139–0.188; 0.139± 0.014 mm wide. Just slightly larger measurements are given by Vieira et al. (2016) for specimens from northeastern Brazil.
Lateral branched spines show a somewhat less regularly symmetrical branching pattern in A. bicornis and a different number of branching tips when fully developed. Less than eight tips per spine are present in A. bicornis (although sometimes more than 10 after Vieira, personal communication, 25.7.2016) while 16 pointed spine tips are usually present in A. emanuelae n. sp. Nevertheless, further observations on a larger number of colonies from different habitats are needed to confidently describe and use this character for discriminating the two species. The development of spines can show a high plasticity in the same species between populations living on different substrata, and/or in environments with distinctive hydrodynamics and other a-biotic parameters, or even in the same colony in relation to micro-environmental changes or predation pressure. This has been documented for other selected electrid bryozoans, such as Electra pilosa (Linnaeus, 1767) ( Bayer et al. 1997) and Arbopercula tenella (Hincks, 1880) from the Mediterranean Sea ( Rosso, 1994a; Rosso in Tessalou et al., 2012).
Two branching spines arising from the proximal opesial edge are also present on the specimen from Saint Francis Island, South Australia collected by Jan Watson, figured by Bock (2016), and considered as close to the variety “ multicornis ”, although exhibiting some differences. This specimen is clearly distinct from both A. bicornis and the present species for the completely different branching pattern of the spines, which are sinuous and asymmetrically bifurcated; the longitudinally elongated opesium, and the dimensions of all gymnocystal spines. Finally, a single stout but simple pointed spine can occur in between the branched ones.
The pattern of contemporaneous budding of paired distal zooids is reminiscent of that described for the inception of the erect phase of A. ramosa by Osburn (1940) and Vieira et al. (2016). It favours the rapid increase in width of colony lobes that A. emanuelae n. sp. shares with Arbocuspis bellula and A. bicornis (see Fig. 22 View FIGURES 22 – 25 and fig. 26 of Vieira et al., 2016). These two species also tend to have zooids arranged in transversal rows, although less markedly than A. emanuelae n. sp. A budding pattern comparable to that observed in the first stages of lobes development in A. emanuelae n. sp. and leading to the arrangement of zooids in nearly transversal rows has been also figured for another representative of the family Electridae , i.e. Aspidelectra melolontha ( Landsborough, 1852) from the British Isles and the North Sea ( Hayward & Ryland, 1998; De Blawue, 2009). This latter species seems to have longitudinal zooidal rows even less connected than A. emanuelae n. sp.
A remarkable character of A. emanuelae n. sp. is the presence of kenozooids, which have been so far not described in any of the species presently assigned to Arbocuspis . Indeed, kenozooids are known from isolated species belonging to other genera within the family Electridae , as is the case with the Lower Miocene Electra triaurata Nikulina & Taylor, 2010 from France, as well as the Recent Einhornia korobokkura ( Nikulina, 2006) and Einhornia venturaensis ( Banta & Crosby, 1994) from Japan and California ( USA), respectively. Kenozooids in E. triaurata seem to serve as connections between zooids of contiguous uniserial branches (fig. 1B in Nikulina & Taylor, 2010), as happens in other uniserial species belonging to electrid genera such as Pyripora d'Orbigny, 1852 (AR, personal observations) and also to other families, such as the calloporid Pyriporoides bathyalis ( Rosso & Taylor, 2002) (see fig. 3A in Rosso & Taylor, 2002). In E. korobokkura , kenozooids simply replace some autozooids in linear chains (see figs 1–2 in Nikulina, 2006). In E. venturaensis , they are small and elevated on the colony surface along the zooidal margins (figs 1–3 in Banta & Crosby, 1994), where they can eventually enlarge to form ‘pseudoancestrular autozooids’ that give rise to subcolonies partly overgrowing the previous colony layer. Kenozooids of A. emanuelae n. sp. seem to be particularly effective in regulating the colony morphology bounding the lateral sides of lobes ( Figs 14–15 View FIGURES 14 – 21 ) and halting their distal growth ( Figs 2 View FIGURES 2 – 4 , 16 View FIGURES 14 – 21 ), also in order to avoid encounters and overgrowths of portions of the same colony ( Figs 17, 19 View FIGURES 14 – 21 ). Indeed, the localised production of kenozooids has been considered relevant in enhancing colony growth flexibility favoring changes in growth direction by McKinney & Jackson (1989). Kenozooids may be common features in bounding colony branches in species with erect growth morphologies, such as in flustriids (see Rosso 1994b). Nevertheless, they usually reinforce branch edges contributing to level the margins with their small sizes, and are often associated with the insertion of new rows and increasing branch width (see Bader & Schäfer, 2004 for Melicerita Milne Edwards, 1836 ). In the encrusting colonies of A. emanuelae n. sp., kenozooids seem to perform opposite functions reducing or maintaining roughly unchanged the branch width. A protective function could be claimed for these kenozooids, which laterally bound colony portions located in slightly depressed, relatively better protected surfaces, possibly to avoid the expansion of the colony on more exposed surfaces. Comparable protective or defensive functions can be suggested for further species where kenozooids along colony margins have been observed, such as for the encrusting multizooidal species Megapora ringens ( Busk, 1856) ( Di Martino & Taylor, 2012).
In addition, isolated kenozoids larger than the marginal ones have been seen in A. emanuelae n. sp. These kenozooids replace autozooids or simply fill space in between them. Isolated or clustered kenozooids often covering large areas have been observed in some multiserial species, such as species of the microporiid genera Mollia Lamouroux, 1816 and Rosseliana Jullien, 1888 (AR, personal observations, and figures in Hayward & McKinney, 2002, their Fig. 13 View FIGURES 9 – 13 E; Chimenz et al., 2014, their Fig. 43e, inter alias). In broken or damaged colony portions, some kenozooids may serve as connections between surviving zooids, and their curving caudae appear to locate zooidal connecting septulae ( Figs 17–18 View FIGURES 14 – 21 ), as in P. bathyalis (see Rosso & Taylor, 2002). Last but not least, some small kenozooids, which lay over zooidal marginal walls, strongly resemble kenozooids described by Banta & Crosby (1994) for E. venturensis . In A. emanuelae n. sp., however, these kenozooids never produced zooid regenerations and subcolonies as observed in A. venturensis . Finally, A. emanuelae n. sp. has kenozooids of different sizes, shapes and presumed functions, as those present in other cheilostomes including species and genera of the family Electridae .
The occurrence in a single species of the genus Arbocuspis (which otherwise has a series of very simple characters, including the mono-to-pauciserial colony growth, and the exclusively terminal budding pattern) of this wide array of kenozooidal polymorphism could be interpreted as a sign of particular adaptation for the environment that A. emanuelae n. sp. colonises. Shallow water sandy bottoms in the Khao Lak area stand in complex hydrodynamic conditions and are subject to recurrent high energy events which cause a constant motion and reorientation of small pebbles on the bottom, leading to a consequent abrasion of lithobionts ( Sanfilippo et al.
2011). Escaping morphologies and the ability to develop colonies in depressed (sheltered) surfaces seem to be functional for surviving in such environments. We suggest that A. emanuelae n. sp. explores this niche locating cavities (Gastrochaenolithes) and confining branches in depressed areas through the development of kenozooids. The species also showed ability to repair damaged zooids and to regenerate zooids through intramural budding, as well as to isolate non functional zooids with closure plates and to reconnect separated colony portions through the insertion of caudate kenozooids. Besides, the pattern of production of new lobes from single zooids along the growing margins of previous lobes, points to the ability of A. emanuelae n. sp. to generate multiphase colonies after possible breakage events.
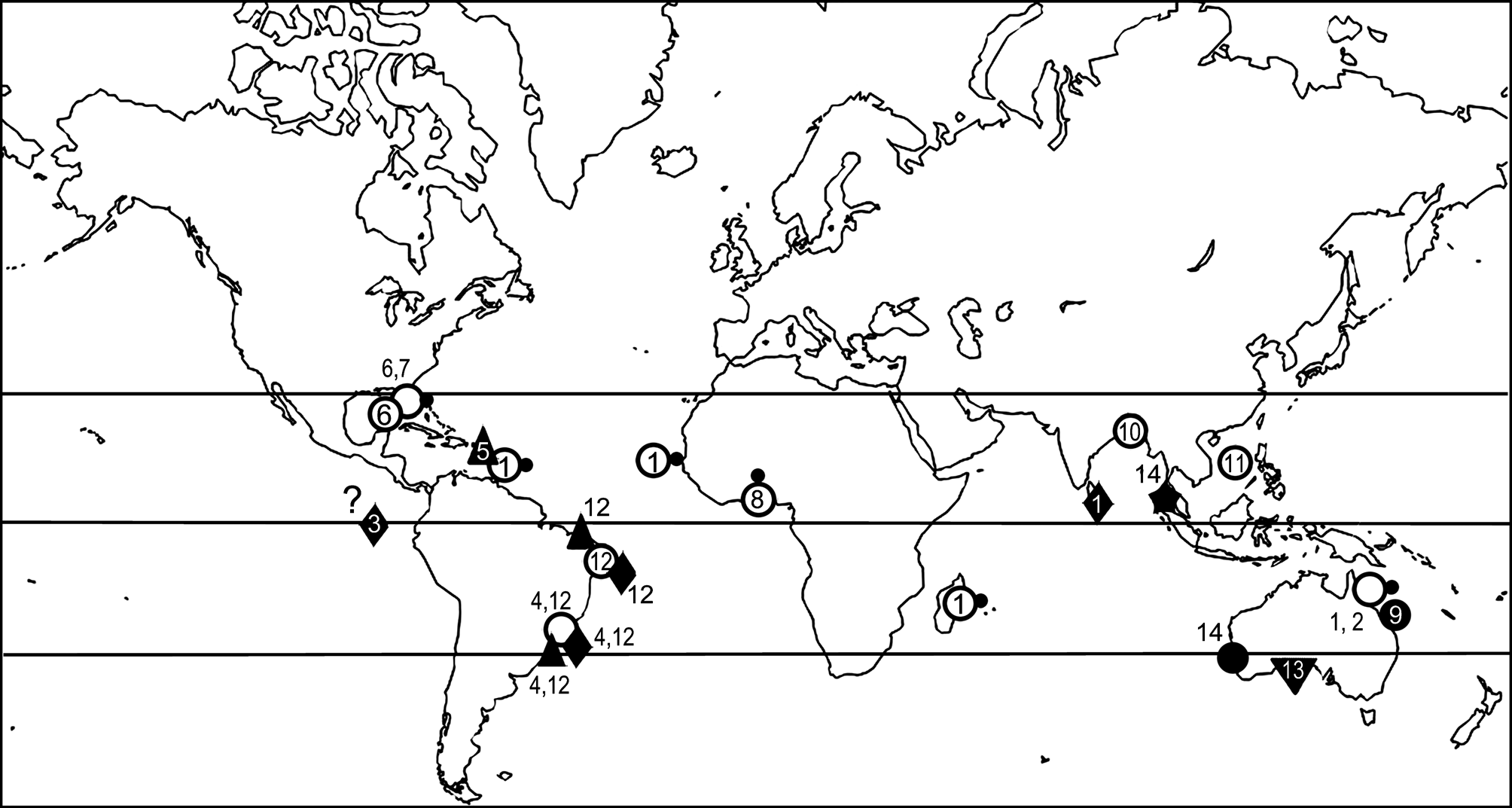
Distribution. This species is presently known exclusively from the Andaman Sea ( Fig. 1 View FIGURE 1 ), from where it has been reported thriving on pebbles in coarse-grained bottoms, at shallow depth.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Malacostegina |
|
Family |
|
|
Genus |