Tremarctos ornatus (F. G. Cuvier, 1825 )
|
publication ID |
https://doi.org/10.1093/mspecies/seab008 |
|
publication LSID |
lsid:zoobank.org:pub:146B87F3-E6A7-4A49-8CFD-140C6DED3DE9 |
|
persistent identifier |
https://treatment.plazi.org/id/BC1287E2-FFCA-C153-FC6A-FA5FFBEAFA9F |
|
treatment provided by |
Felipe |
|
scientific name |
Tremarctos ornatus (F. G. Cuvier, 1825 ) |
| status |
|
Tremarctos ornatus (F. G. Cuvier, 1825) View in CoL
Spectacled Bear
Ursus ornatus F. G. Cuvier, 1825:57 View in CoL . Type locality “Cordilléres du Chili,” restricted to “las montañas al Este de Trujillo, departamento de la Libertad, Perú,” by Cabrera (1957:242).
Ursus frugilegus Tschudi, 1844:90 View in CoL . Type locality unknown; stated as Perú, probably near Lima by Allen (1942:396; see “Nomenclatural Notes”).
Ursus fructilegus Schinz, 1845:34 . Type locality “In Peru.”
Helarctos ornatus Gray, 1864:698 . Type locality “South America, Cordilleras.”
Ursus nasutus Sclater, 1868:72 View in CoL , Plate VIII. Type locality not given (description based on a purchased specimen); stated as Simitarra River, Upper Magdalena, Colombia by María (1924).
Nearctos ornatus: Gray, 1873:182 . Name combination.
Tremarctos ornatus: Gill, 1874:15 View in CoL . First use of current name combination.
Tremarctos ornatus majori Thomas, 1902: 216 . Type locality “Southern Ecuador, probably the province of Azuay.”
Ursus ornatus thomasi Hornaday, 1911:748 . Type locality “Andes of southern Colombia.”
Tremarctos lasallei María, 1924:115 View in CoL . Type locality “ región de Arauca,” Colombia.
CONTEXT AND CONTENT. Context as for genus. Tremarctos ornatus View in CoL is considered monotypic ( Kitchener 2010).
NOMENCLATURAL NOTES. The monophyletic origin of Ursidae with the giant panda ( Ailuropoda melanoleuca ) as a sister clade is widely accepted ( Agnarsson et al. 2010). Tremarctos ornatus and its related fossil forms are placed in a separate subfamily, Tremarctinae or “short-faced” bears, but this taxonomic classification is not universally accepted ( Thenius 1976; Wilson and Reeder 2005; Garshelis 2009). Five specific or subspecific names have been applied to T. ornatus , based on claw length, facial markings, and body proportions. Allen (1942) refers to U. frugilegus, Tschudi 1844:90 , U. ornatus thomasi Hornaday 1911:748 , and T. lasallei María 1924:115 as synonyms of T. ornatus majori . Allen believed U. frugilegus to be inseparable from the species described by Cuvier. In a review of the materials collected by J. Tschudi in Peru (1838–1942) it was concluded that Tschudi’s description of U. frugilegus was based on discussions with local hunters rather than an actual specimen ( Serrano-Villavicencio et al. 2020). Tschudi made no reference to a collected specimen, only commenting that “due to the local climate, preserving skins of this species was a difficult task”; as of 2020 no type material had been located ( Serrano-Villavicencio et al. 2020:916).
Because all geographical forms were described by different authors based only on physical variations, Cabrera (1957) assigned all geographical forms as inadmissible, referring all variations to Tremarctos ornatus Cuvier, 1825 . T. ornatus , is treated as a monotypic species, although considerable phenotypic variation is present within the species ( Krause et al. 2008; Agnarsson et al. 2010; Kitchener 2010).
The etymological origin of the generic name came from Trema (Greek meaning hole) and arktos (Greek meaning bear) and refers to an unusual hole in the humerus. The specific name, ornatus (Latin meaning dress— Gotch 1979), refers to the lightcolored patches of fur surrounding the eyes, muzzle, chest, and throat. These characters contrast with the black-colored fur, which in some cases creates the appearance of eyeglasses (also called spectacles), and serves as the basis for one of its common English names: spectacled bear ( Pérez-Torres 2001), whereas the other common English name is Andean bear (Velez-Liendo and García-Rangel 2017).
In several Andean indigenous cultures, the species is known as “ Jukumari,” which means bear (Paisley and Saunders 2010). In Quechua, the word uku means hole, so the word probably was used as the “bear with holes in the eyes” ( Pérez-Torres 2001). Throughout its range, T. ornatus is known by other common names, such as Mashíramo, Oso Frontino, Oso Salvaje, Oso Real, Uí, Oso Careto, Iznachi, Manaba, Puca mate, and Ucucu ( Mondolfi 1989; Goldstein 2002; Castellanos et al. 2016a).
DIAGNOSIS
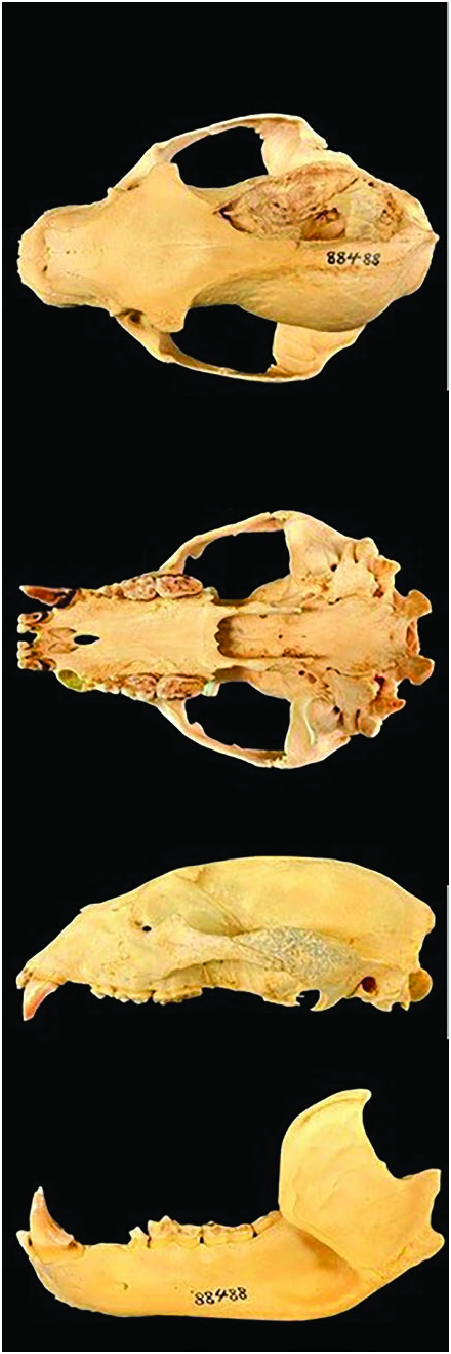
Tremarctos ornatus ( Fig. 1 View Fig ) is a medium-sized bear (head– body length 1,190 –1,740 mm — Mondolfi 1971; Bininda-Emonds 1998) and is larger than the sun bear ( Helarctos malayanus , head–body length 1,000 –1,400 mm —Fitzgerald and Krausman 2002), but is similar in size to the American black bear ( Ursus americanus , head–body length 1,430 –1,570 mm — Lariviere 2001). T. ornatus has the shortest muzzle ( 9–129 mm) of all bear species and the shortest mean (± SD) skull length of any bear species (201 ± 6.45 mm) only comparable with the sun bear (213 ± 13.15 mm —Christiansen 2007; Stucchi and Figueroa 2013). Claws are short on front and rear feet ( Peyton 1980, 1999; Garshelis 2009) in comparison with the sun bear that has strongly curved and pointed claws (Fitzgerald and Krausman 2002). The mandible of T. ornatus has a unique trait not present in other ursid species: a deep fossa called the premasseteric fossa ( Mondolfi 1983).
GENERAL CHARACTERS
Tremarctos ornatus is a medium-sized bear with rounded ears, a plantigrade stance, front limbs longer than the hindlimbs, nonretractable curved claws, and a short tail ( Mondolfi 1971; Nowak 1999; Peyton 1999; Garshelis 2009; García-Rangel 2012). Pelage is black to dark red-brown with dense, long, and coarse fur ( Mondolfi 1971; Garshelis 2009). Body coat color varies from black or blackish brown to blackish red ( Mondolfi 1989; Nowak 1999; Garshelis 2009). Individual bears usually exhibit white, yellowish or creamy marks around the eyes that continue to the muzzle, cheeks, throat, neck, and chest ( María 1924; Allen 1942; Garshelis 2009; García-Rangel 2012). These marks are highly variable across the geographic range, not only in color but also size, and can range from prominent to completely absent ( Allen 1942; Mondolfi 1971; Garshelis 2009; Réyes et al. 2017), and they are the most distinctive phenotypic character for T. ornatus . When present, facial marks are more conspicuous in young, whereas in adults the facial marks can become thinner with age; they do not indicate family relationships between individuals ( Van Horn et al. 2014b, 2015).
As with all bears, T. ornatus is sexually dimorphic; adult females are 67% as large as males ( Mondolfi 1989; Garshelis 2009; García-Rangel 2012). Furthermore, male skulls are larger than female skulls (skull length [mean ± SD] males = 236.8 ± 12.0 mm; females = 202.2 ± 21.8 mm) and males have a well-developed lambdoid crest and a prominent sagittal crest that is absent or reduced in females ( Fig. 2 View Fig ; Emslie 1995; Stucchi and Figueroa 2013).
Head–body length for T. ornatus varies between 1,100 and 2,200 mm ( Bininda-Emonds 1998; Nowak 1999; Garshelis 2009; García-Rangel 2012). Mean external measurements (mm; ± SD; n — Mondolfi 1971) for males from Venezuela were: total length (1,485 ± 360; 2), tail length (100; 1), ear length (100; 1), and mean weight (91 ± 68 kg; 2). Colombian males in the wild have a mean head–body length of (1,350 ± 219; 3), tail length (733 ± 20; 3), ear length (103 ± 18; 3), and weight (105 ± 60 kg; 3). Females have a mean head–body length (1,260 ± 28; 2), tail length (80 ± 14; 2), ear length (100; 1), and mean weight (37.5 ± 2 kg; 2— Rodríguez et al. 2013). Head–body length from one adult male from Ecuador was 1,312 mm, tail length 150 mm, and ear length 64 mm. Head– body length from one juvenile female in Ecuador was 270 mm and ear length 52 mm ( Tirira 2009). T. ornatus from Bolivia weighed 34 kg (male subadult) and 30 kg (“undersized” adult male), but no other measurements were reported ( Paisley 2001). No information is available for T. ornatus in Peru, except that Peyton (1980) estimated weights between 20 and 120 kg.
Derived from the oldest fossil specimen and four modern T. ornatus , skull and mandible measurements from T. ornatus (mm; mean ± SD — Stucchi et al. 2009) were: condylobasal length 223.16 ± 14.02, median palatal length 103.2 ± 7.9, palatal breadth at M2 41.28 ± 2.72, breadth at the labial C (upper canine) alveoli 57.62 ± 2.31, breadth at the lingual C alveoli 34.4 ± 2.06, zygomatic breadth 159.82 ± 6.9, frontal breadth 84.74 ± 8.45, least breadth between the orbits 62.22 ± 6.98, facial length 114.44 ± 2.61, mandible length 164.54 ± 6.66, height of the coronoid process 95.18 ± 7.87, basal length of the coronoid process 48.65 ± 6.64, height of the horizontal ramus at M2 35.58 ± 1.53.
Tremarctos ornatus lacks a diastema, unlike the insectivorous (e.g., the sloth bear, Melursus ursinus ) and carnivorous (e.g., the polar bear, Ursus maritimus ) bears ( Figueirido et al. 2009; Stucchi and Figueroa 2013). The dental formula is i 3/3, c 1/1, p 4/4, m 2/3, total 42 ( Ramsay 2003; García-Rangel 2012) and includes the shortest incisors in the Ursidae . The canines are bladed and an extra lateral cusp, located between the trigonid and T. ornatus in comparison with human tarsal ligaments, detailing that the tarsus of T. ornatus is more flexible but is a less stable structure than in humans. T. ornatus has an enlarged radial sesamoid called “false thumb,” a feature shared with the panda. The function of this thumb is still debated ( Salesa et al. 2006).
taloned, occurs on m1 ( Emslie 1995; Sacco and Van Valkenburgh 2004; Stucchi and Figueroa 2013).
The humerus of T. ornatus can be recognized by the presence of a fistula situated above the internal epicondyle (Cabrera and Yepes 1940). Davis (1958) described the tarsal ligaments of
DISTRIBUTION
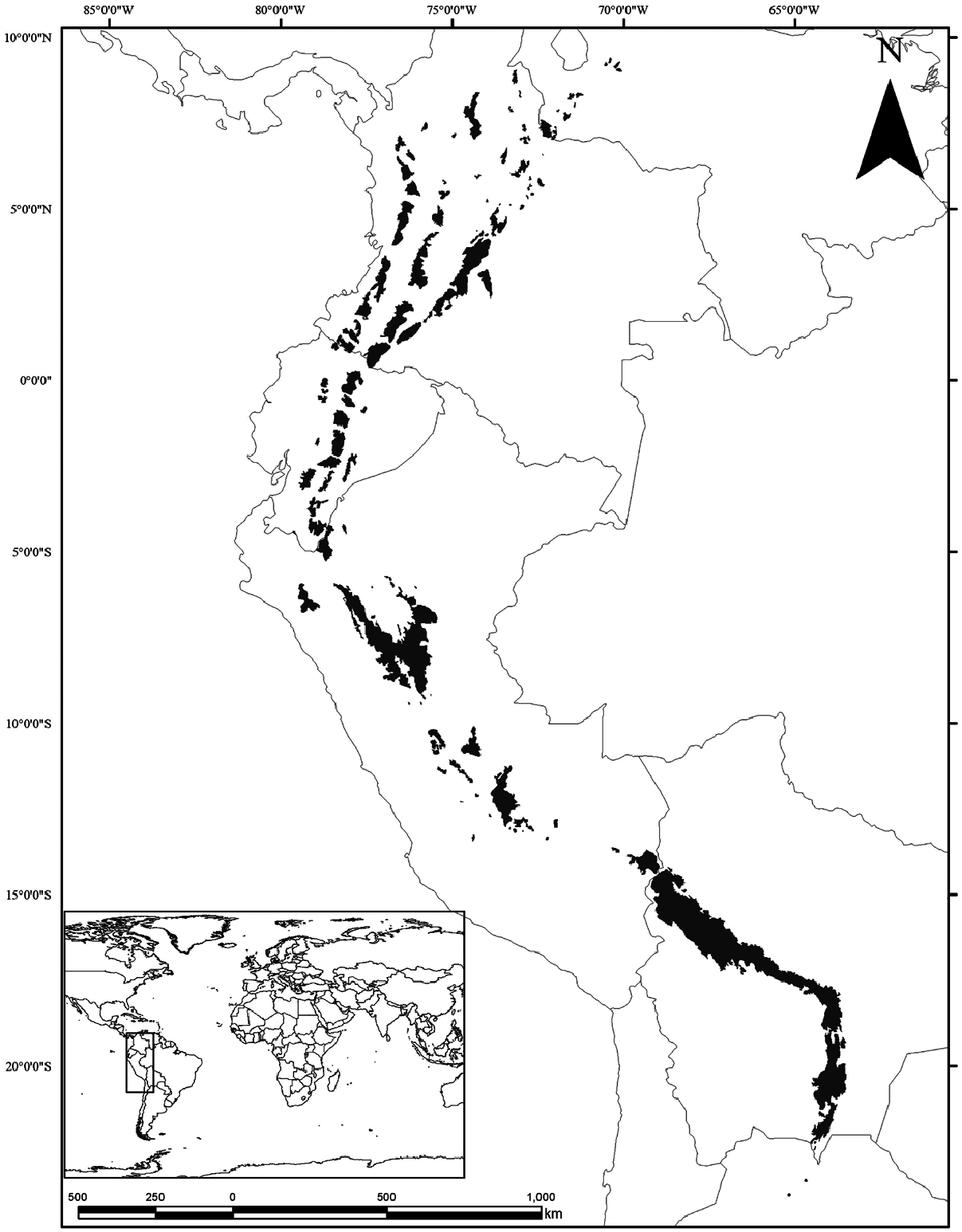
Tremarctos ornatus is distributed in the South American tropical Andes region ( Fig. 3 View Fig ) in an elevational range between 200 and 4,250 m ( Peyton 1980, 1981, 1999; García-Rangel 2012). During the Pleistocene, the distributional range was larger ( Stock 1950). Currently the distribution is an elongated and narrow range that is about 200–650 km wide and 4,600 km long ( Peyton 1999; Kattan et al. 2004; García-Rangel 2012), including six countries: Venezuela, Colombia, Ecuador, Perú, Bolivia, and Argentina ( Mondolfi 1989; Peyton 1999; Garshelis 2009; García-Rangel 2012; Cosse et al. 2014; Velez-Liendo and García-Rangel 2017). T. ornatus potentially occurs in Panama, but there are no records to support its presence ( Allen 1942; Hershkovitz 1957; Mondolfi 1971, 1989; Goldstein et al. 2008).
In Venezuela, T. ornatus is widely distributed in the Andes in discontinuous forested mountain areas in the western portion of the country ( Lara, Táchira, Mérida, Portuguesa, Zulia, and Trujillo States— Osgood 1911; Mondolfi 1971, 1989; Goldstein 1992; Bisbal 1993; Soriano et al. 1999; SánchezMercado et al. 2014).
In Colombia, T. ornatus has been reported from three biogeographic regions (Caribbean, Andes, and Pacific) and 20 departments ( Alberico et al. 2000; Jorgenson and Sandoval-A 2005; Solari et al. 2013; Vela-Vargas et al. 2014; Cáceres-Martínez et al. 2016; Rodríguez et al. 2019a). In Ecuador, T. ornatus is distributed in the Sierra, High Amazon, and Andes regions, and is present in both ranges of the Andes and the Condor and Cucutú subranges ( Suarez 1988; Castellanos 2011; Zapata-Ríos and Branch 2016). In Peru, T. ornatus is found in all three ranges of the Andes, with 973 records of presence distributed in 17 departments on an elevation range between 400 and 3,692 m (Márquez and Pacheco 2010; Falconi 2019; Falconi et al. 2020). In addition to Andean forest and páramos, T. ornatus lives in dry and humid tropical areas of less than 1,000 m elevation ( Figueroa 2012; Figueroa and Stucchi 2013; Filipczyková et al. 2016; Appleton et al. 2018).
In Bolivia, T. ornatus is known from four departments: La Paz, Santa Cruz, Chuquisaca Cochabamba, and Tarija (Salazar and Anderson 1990; Anderson 1997; Martínez et al. 2008; Albarracín et al. 2013). The southern distributional extent of T. ornatus is the southern portion of Tarija department in Bolivia, coinciding with the northwestern border of Argentina (Vargas and Azurduy 2006). Derived from 18 unconfirmed sightings in northwestern Argentina, del Moral and Bracho (2005) suggested the presence of T. ornatus in the country. After several years of debate (Del Moral and Bracho 2005, 2009; Rumiz et al. 2012), Cosse et al. (2014) confirmed its presence in Jujuy and Salta provinces in northern Argentina using noninvasive samples (hair and feces) to generate genetic identification.
The potential presence of T. ornatus in Panama is based on a skull and traditional knowledge from indigenous communities ( Hershkovitz 1957). Later, field surveys at the Serranía de Pirre (along the border with Colombia) were unable to confirm the presence of T. ornatus in Panama ( Goldstein et al. 2008).
FOSSIL RECORD
The earliest tremarctine bears are known from late Miocene fossils in North America (Tedford and Martin 2001). Four genera of tremarctines are currently recognized: Plionarctos (extinct, two species), Arctodus (extinct, two species), Arctotherium (extinct, five species), and Tremarctos with two species, including the only extant species, T. ornatus ( Soibelzon et al. 2005, 2008; Mitchell et al. 2016). The most complete fossil specimen includes skull, mandible vertebrae, ribs, hindlimbs, and forelimbs and the oldest record of T. ornatus , an adult male from Chaquil Cave, Amazonas department, Peru, dated 5,980 ± 50 radiocarbon years ago, is reported by Stucchi et al. (2009). Additional records are from two archeological sites in Colombia dated from 4,030 to 2,725 years ago, in Cundinamarca department ( Urrego-Correal 1990), and one fossil record from Peru dated from 1,500 years ago in Cajamarca department ( Florez 1975). These fossil records consist only of small pieces of bones (ulna, canine, metacarpus), but are the only specimens known. Skeletal remains from 700 BCE to 250 CE were excavated at La Chimba site in Ecuador (Stahl and Athens 2001).
Tremarctine is considered monophyletic by Mitchel et al. (2016), but mtDNA phylogenetic analyses suggest that Arctodus (North America) and Arctotherium (South America) lineages are not sister taxa (from a monophyletic clade), as suggested by Trajano and Ferrarezzi (1995). Tremarctos and Arctotherium are hypothesized to be sister taxa, diverging in the Pliocene. The estimated divergence estimation for Arctodus and Tremarctos is 5.66 million years ago ( Krause et al. 2008). The Plionarctos linage is ancestral to Arctodus , Arctotherium , and Tremarctos but formed as a paraphyletic stem group (Tedford and Martin 2001).
Based on three fossils of an ursine bear from the late Miocene in Nebraska ( United States) described as Aurorarctos tirawa gen. et sp. nov. (two mandibles, 14 isolated teeth, and one partial humerus), Jiangzuo and Flynn (2020) hypothesized the monophyly of tremarctine bears, but the clade name Arctotheriini is used to represent the crown + stem-group lineages. Tremarctinae is not eliminated but is just not used as a clade name because Jiangzuo and Flynn recognize Ursinae as the group containing not only the Ursus lineages (Ursini), but the tremarctines and the early ursine Aurorarctos .
FORM AND FUNCTION
Form. —The skull of Tremarctos ornatus is wide and heavy with thick boney walls and high bone density ( Fig. 2 View Fig ; Christiansen 2008a; Stucchi and Figueroa 2013). These features could be a response to an omnivorous diet with high consumption of plants (Christiansen and Wroe 2007; Christiansen 2008b; Jiangzuo and Flynn 2020). T. ornatus possesses blade-shaped canines (bite force [mean ± SD] at the canine tip = 607.52 ± 143.77 N) with enlarged molars, characteristic traits of omnivorous species (Sacco and Van Valkenburgh 2004; Christiansen and Wroe 2007). The zygomaticomandibularis of T. ornatus is relatively larger than in other bear species showing a moderate bite force in comparison with specialized bears, such as the giant panda ( Ailuropoda melanoleuca — Davis 1955; Súarez 1985; Peyton 1999; Christiansen and Wroe 2007; García-Rangel 2012). In contrast, the superficial masseter is smaller than in other carnivores and differs from flesh-eating species in having a shorter, concave-shaped mandible that allows grinding and cutting movements ( Davis 1955; Figueirido et al. 2009). These bone, teeth, and muscle characteristics are correlated with a predominantly herbivorous diet ( Davis 1955; Christiansen and Wroe 2007; Figueirido et al. 2009; García-Rangel 2012). The tongue is wide ( 35 mm). The lips are large and flexible, as in all Ursidae ( Davis 1955; García-Rangel 2012). Brain weight from a captive individual was 240 g ( Hirata 1987).
In locomotion, T. ornatus uses only five ligaments in the talocrural articulation and a well-developed, interarticular meniscus ( 14 mm long by 3 mm wide), which implies lateral thrust of the astragalus against the fibula in strong inversion, such as during climbing ( Davis 1958). The radial sesamoid is mediolaterally flattened, whereas the distal tip is scarcely developed, like a blunt protuberance ( Salesa et al. 2006).
Length of the baculum is 10.47 mm, height at the level of the basal end is 6.1 mm, height at the level of the distal end is 3.8 mm, and maximum width is 7.9 mm; these measurements were derived from one subadult male killed in Venezuela ( Mondolfi 1983). The baculum is nearly straight, gradually tapered from base to tip, and ends in a slightly enlarged blunt tip ( Mondolfi 1983).
The adaptive value of the large white marks on the muzzle, upper chest, and neck, and the large white circles around the eyes is unknown; however, these regions might modulate intraspecific aggression and dominance ( Caro 2009). All bears have epipharyngeal pouches. Based on the examination from one individual of T. ornatus (Forstenpointner and Weissengruber 2000) , these are a single pouch and two smaller sacs that are outfoldings of the lateral pharyngeal wall. It is thought that these could play a role in the production of vocalizations via the movement of the neck and enable management of the air column (Forstenpointner and Weissengruber 2000; Weissengruber et al. 2001).
Ovaries in Tremarctos ornatus are located on the sublumbar region anterior of the kidneys (Enciso and Vásquez 2007). The mean length of the right ovary is 18.5 ± 2 mm ( n = 2), 11.5 ± 2 mm width ( n = 2), and 9 ± 1 mm thickness ( n = 2). The mean length of the left is 19 mm ( n = 2), width is 10.5 ± 2 mm ( n = 2), and thickness is 8.5 ± 0.7 mm ( n = 2— Lengwinat et al. 2001; Enciso and Vásquez 2007). Macroscopically, the placenta (placenta discoidalis) is disk-shaped with a length of 120 mm, width of 95 mm, thickness of 5 mm, and mass of 55.4 g ( Michel et al. 1983).
Scrotum length and height are 465.0 and 629.5 mm, respectively. Testes are oval oblique to the dorsocaudal major axis (right testis 386 by 241.5 mm, left testis 476 by 241.5 mm —Sanchez- Arbouin and Nassar-Montoya 1997). Sperm measurements (mean ± SD) were: head length 5.34 ± 0.15 µm, head width 3.72 ± 0.05 µm, area of head 16.84 ± 0.55 µm 2, head perimeter 16.07 ± 0.32 µm, acrosome 67.70 ± 3.38%, area if intermedium piece 2.06 ± 0.22 µm 2, and intermedium piece width 0.95 ± 0.09 µm ( Enciso et al. 2006). Sperm motility in males was recorded between 50% and 70%, with 10–15% abnormalities (SanchezArbouin and Nassar-Montoya 1997; Enciso et al. 2006).
Function.— The polar bear and Tremarctos ornatus have the lowest means for corpuscular volume (MCV) of red blood cells and the highest blood albumin level of all bears ( Seal et al. 1970). Based on a sample of 62 bears of all species, T. ornatus possesses the smallest corpuscular volume with an average of 55 fl ( n = 40— Seal et al. 1967; Castellanos et al. 2010). Blood cell size could be a response to seasonal changes and food availability for T. ornatus and the polar bear ( Seal et al. 1967; Castellanos et al. 2010).
Male individuals ( n = 23) had higher mean serum protein levels ( 155.6 g /l) than did females ( n = 23, 132.8 g /l— Castellanos et al. 2010). Hematological values (mean ± SD) for combined sexes of captive, reintroduced, and wild animals were: cholesterol 7.98 ± 1.97 mmol/l ( n = 33), total protein 77.30 ± 15.30 g /l ( n = 27), triglycerides 7.33 ± 1.64 mmol/l ( n = 34), blood urea nitrogen 4.77 ± 1.51 mmol/l ( n = 29), glutamic oxalic transaminase 30.23 ± 20.68 U/l ( n = 38), glutamic pyruvic transaminase 21.43 ± 19.41 U/l ( n = 39), alkaline phosphatase 97.57 ± 58.21 U/l ( n = 31), calcium 1.87 ± 0.28 mmol/l ( n = 32), phosphorus 1.68 ± 0.70 mmol/l ( n = 35), glucose 3.56 ± 1.46 mmol/l ( n = 18), urea 9.85 ± 4.48 mmol/l ( n = 38), hematocrit 0.43 ± 0.05 l/l ( n = 45), hemoglobin 144.45 ± 20.89 g /l ( n = 46), leukocytes 9.11 ± 2.98 × 109 /l ( n = 44), erythrocytes 7.87 ± 1.52 × 1012 /l ( n = 37), segmented leukocytes 6.47 ± 0.94 × 109 /l ( n = 45), lymphocytes 2.20 ± 0.77 × 109 /l ( n = 44), monocytes 0.13 ± 0.13 × 109 /l ( n = 46), eosinophiles 0.16 ± 0.22 × 109 /l ( n = 43), basophiles 0.01 ± 0.05 × 109 /l ( n = 43), band cells 0.027 ± 0.11 × 109 /l ( n = 40), mean cellular hemoglobin concentration 334.17 ± 43.10 g /l ( n = 36), mean cellular hemoglobin 18.33 ± 3.03 pg ( n = 38— Nassar-Montoya et al. 1997; Castellanos et al. 2010).
ONTOGENY AND REPRODUCTION
Length at birth is 225–280 mm and weight is 300–500 g ( Saporiti 1949; Roth 1964; Dathe 1967; Castellanos et al. 2016a). Neonates are black and toothless at birth, with closed eyes. Eyes open completely by day 31 ( Saporiti 1949). The only record of growth rate for young Tremarctos ornatus was presented by Saporiti (1949) as 50 mm /day.
Most of the information available for reproduction of T. ornatus has been obtained from captive individuals ( Saporiti 1949; Roth 1964; Gensch 1965; Bloxam 1977; Michel et al. 1983; Kuhme 1991; Lengwinat et al. 2001; García-Rangel 2012). T. ornatus is a polyestrous species with facultative seasonal reproduction ( Saporiti 1949; Mondolfi 1971; Spady et al. 2007; Enciso 2013) and is capable of embryonic diapause ( Lengwinat et al. 2001; Knauf et al. 2003; Enciso 2013). After diapause, gestation length is short and difficult to calculate ( Michel et al. 1983; Rosenthal 1987; Spady et al. 2007; García-Rangel 2012).
Captive females present 3–4 phases of ovarian activity per year ( Enciso 2013). Wild females have an interval of 9 months between estrous cycles with a maximum of three cycles in 24 months ( Spady et al. 2007). Duration of estrus is estimated at 5 days ( Spady et al. 2007) but can vary depending on the latitude and associated photoperiod cycle of the facility ( Knauf et al. 2003). Captive females from Brazil and Colombia had estrous cycles that lasted 3–10 days (Sanchez-Arbouin and NassarMontoya 1997; Enciso 2013).
Gestation varies from 120 to 254 days ( Saporiti 1949; Gensch 1965; Bloxam 1977; Kuhme 1991; Castellanos 2015). Castellanos (2015) reported the shortest gestation period of 120– 125 days, similar to the period reported for the sun bear ( Frederick et al. 2012). Litter size varies from 1 to 3 young. Sexual maturity in females is attained at 4–7 years of age ( Saporiti 1949; Dathe 1967; Bloxam 1977; Rosenthal 1987).
Tremarctos ornatus displays seasonal reproductive activity in wild individuals that corresponds to resource availability, but in captive individuals is associated with photoperiod ( Appleton et al. 2018). Births are concentrated in autumn in tropical latitudinal zones (< 23.5°N or S), whereas in mid-temperate latitudinal zones (35– 55°N or S) most births occur during the winter ( Peyton 1980; Rosenthal 1987; Spady et al. 2007; García-Rangel 2012; Appleton et al. 2018).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tremarctos ornatus (F. G. Cuvier, 1825 )
| Vela-Vargas, I Mauricio, Jorgenson, Jeffrey P, González-Maya, José F, Koprowski, John L, Rose, Robert K, Owen, Pamela R & Hamilton, Meredith J 2021 |
Tremarctos lasallei María, 1924:115
| MARiA, A. 1924: 115 |
Ursus ornatus thomasi
| HORNADAY, W. 1911: 748 |
Tremarctos ornatus majori
| THOMAS, O. 1902: 216 |
Tremarctos ornatus : Gill, 1874:15
| GILL, T. 1874: 15 |
Nearctos ornatus :
| GRAY, J. E. 1873: 182 |
Ursus nasutus
| SCLATER, P. L. 1868: 72 |
Helarctos ornatus
| GRAY, J. E. 1864: 698 |
Ursus fructilegus
| SCHINZ, H. 1845: 34 |
Ursus frugilegus Tschudi, 1844:90
| ALLEN, G. 1942: 396 |
| TSCHUDI, J. 1844: 90 |
Ursus ornatus F. G. Cuvier, 1825:57
| CABRERA, A. 1957: 242 |
| CUVIER, F. 1825: 57 |