Arrhopalites
|
publication ID |
https://doi.org/10.5281/zenodo.186465 |
|
DOI |
https://doi.org/10.5281/zenodo.5618652 |
|
persistent identifier |
https://treatment.plazi.org/id/BA7487DE-EF7B-FFBE-FF28-4444FD320AA3 |
|
treatment provided by |
Plazi |
|
scientific name |
Arrhopalites |
| status |
|
Arrhopalites caecus- and pygmaeus- group of species or separate genera?
Stach (1945: 7) composes the species of genus Arrhopalites into two groups: caecus -group with anterior distal spine on dens and small cuticular spines on anal valves, and pygmaeus -group without anterior distal spine on dens and spines on anal valves. Since Stach’s work several species close related to A. caecus and seemed as belonging to the caecus -group but without one of the mentioned remarkable characters have been described. Consequently Bretfeld (1999: 66) redefined caecus -group: “Dens with 1 anterior distal spine or posterior side of Abd VI with small spines or projections in addition to normal setae, also both characteristics present”.
This definition seems somewhat strain: one can suppose that if inside this group the tendency of secondarily losing of cuticular spines on Abd VI or transforming anterior distal spine on dens into ordinary seta occurs, why both of these characters can not occur together (without cuticular spines on Abd VI and without anterior distal spine on dens but belonging to the caecus -group). In other words, existence of species from the caecus -group but without any distinctive characters defined for this group is supposed to be quite possible. For example, Arrhopalites pukouensis Wu & Christiansen, 1997 satisfies this condition. Accordingly, understanding the caecus -group as a monophyletic composition requires looking for more constant synapomorphies and taking into account a wider complex of characters.
A. caecus - and pygmaeus -group problem was also discussed by Zeppelini (2004, 2006) who supports the monophyly of the caecus -group. He considers the presence of cuticular spines on anal valvae as synapomorphic to the caecus -group and suggests that the spines in the dorsal and ventral flaps have evolved independently from each other, besides, these spines may be secondarily lost. He also considers the presence of 5 rows of setae on anterior surface of dens as a feature typical for the caecus -group. After Zeppelini (2004) the pygmaeus -group is supported by the presence of only 4 rows on the anterior surface of dens, and seta Ie on the posterior surface is strongly spinelike. However, as it will be shown below, the rule of 4 anterior setal rows in pygmaeus -group and 5 in caecus -group is not always obvious. At last, it was mentioned that the “genus Arrhopalites can be undoubtfully recognized by its metatrochanteral organ and seta E1 spinelike on dens” ( Zeppelini 2004).
Further looking for distinctiveness between caecus and pygmaeus groups based mainly on the material from Eastern Europe (23 species) and on information from literature sources on genus Arrhopalites allows to expand and revise previous definitions of these groups. Moreover, I believe that the newly indicated characters together with characters found before are sufficient for splitting genus Arrhopalites into two separate genera. These characteristics are listed below.
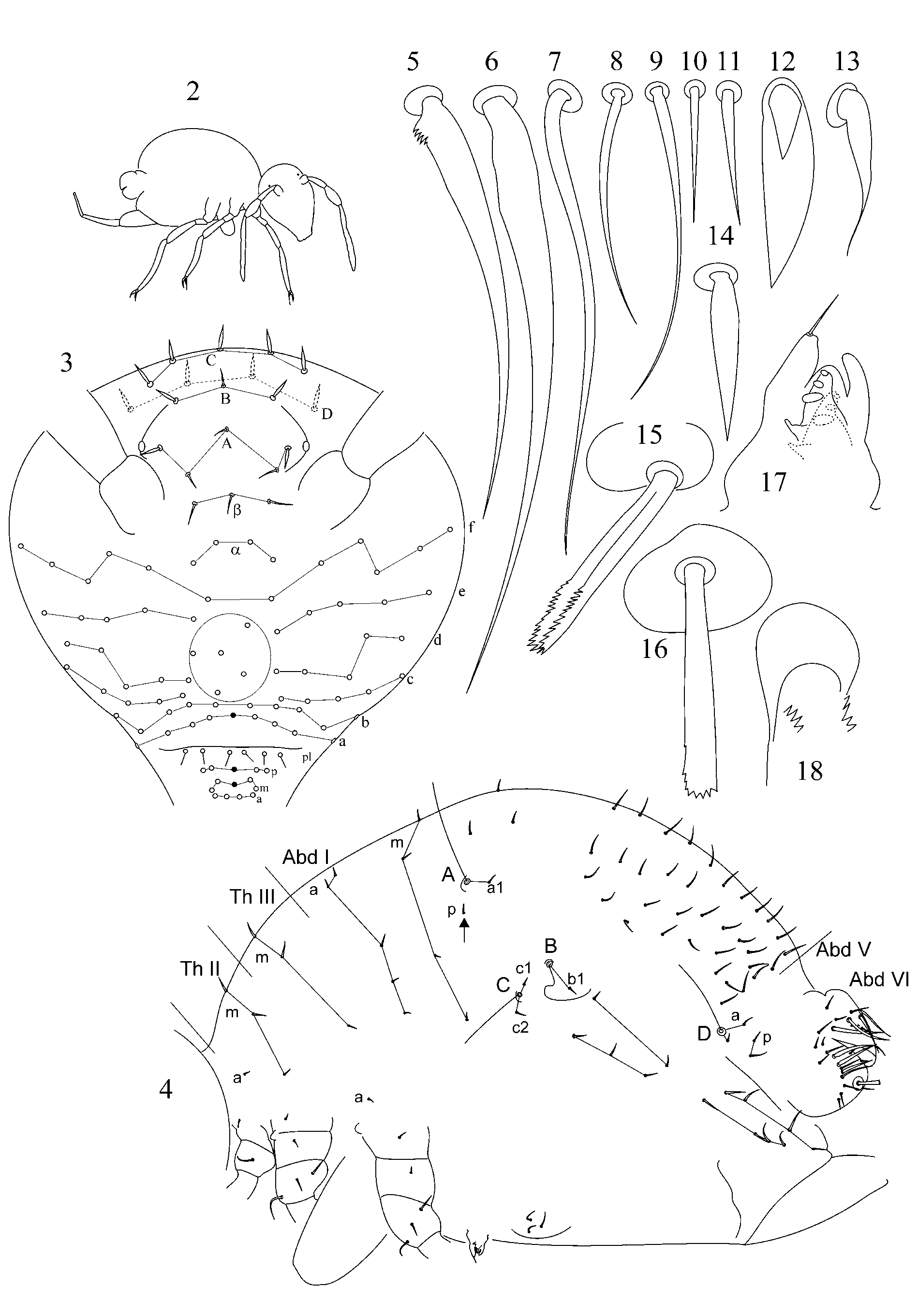
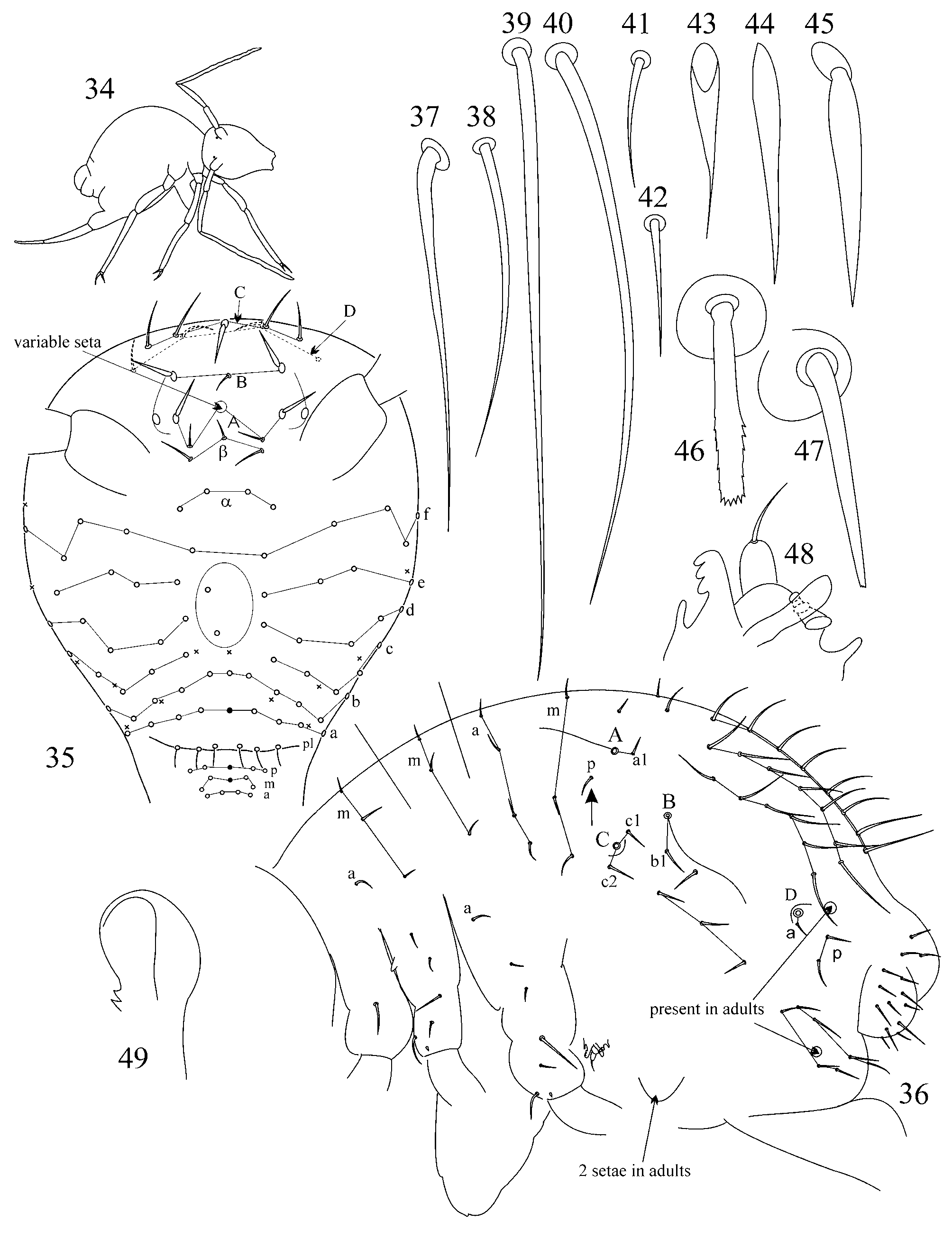
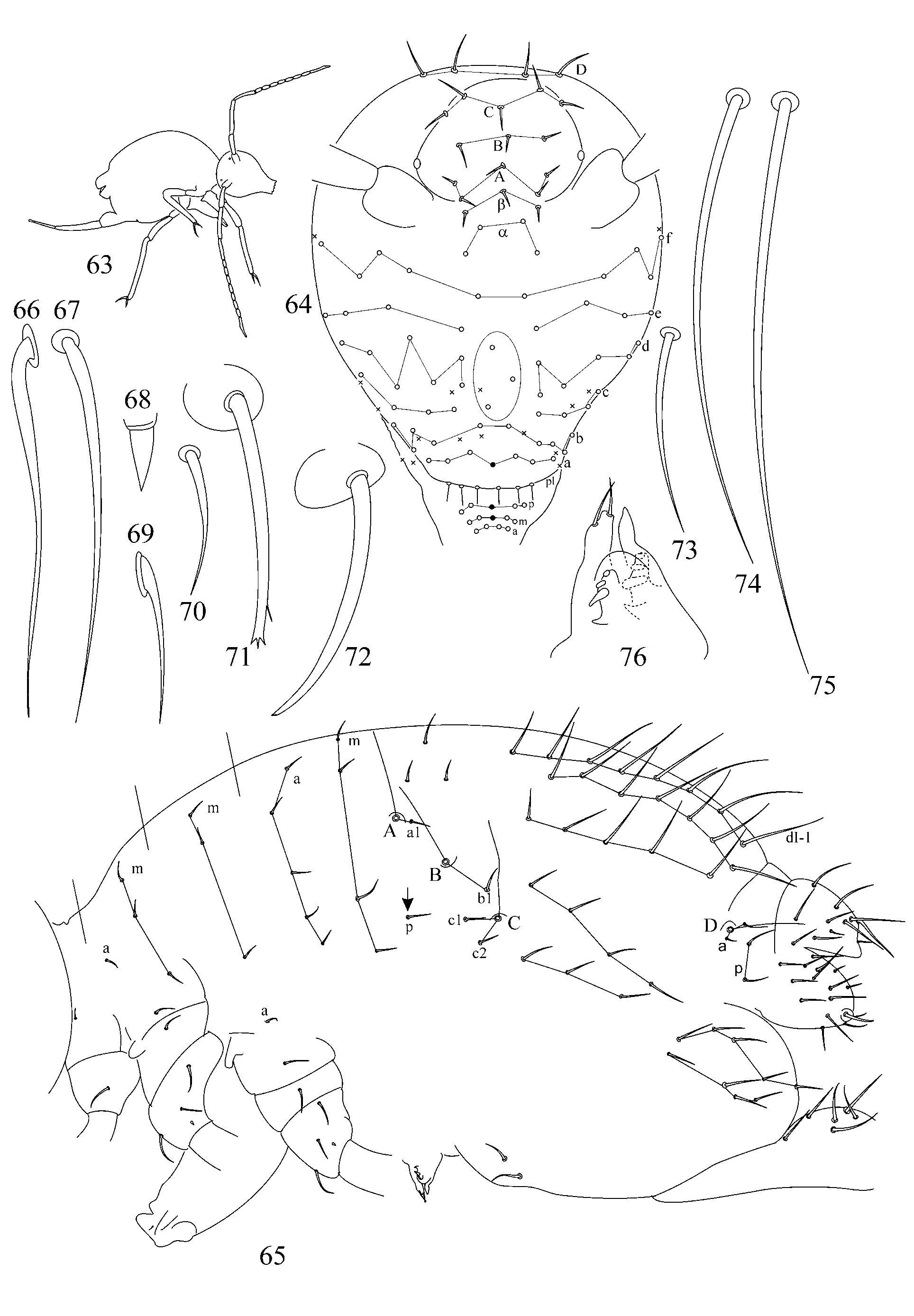
1. Chaetotaxy of great abdomen. Trichobothrial pattern in Symphypleona is a feature of high taxonomic value even on suprageneric level ( Richards 1968; Betsch & Waller 1989, 1994; Bretfeld 1999). For the genus Arrhopalites the pattern was defined as triangular or forming an angle ABC opening anteriorly ( Richards 1968; Betsch 1980; Betsch & Waller 1989; Christiansen & Bellinger 1998; Bretfeld 1999), although, it was rarely mentioned and drawn in species descriptions. Concerning Arrhopalites Betsch and Waller (1989) in the work devoted to trichobothrial arrangement in Symphypleona describe only Arrhopalites gr. pygmaeus pattern. However, the examination of trichobothrial complex (localization of trichobothria and associated setae) in species of caecus and pygmaeus groups shows that the patterns are different.
1.1. Pattern of caecus type ( Figs 4 View FIGURES 2 – 18 , 36 View FIGURES 34 – 49 ): trichobothria ABC form an angle towards 90º, trichobothrium B stands remarkably out of line AC. Additional characters: single p seta of p-row of Abd I is located above the level of trichobothrium B (marked with arrow); seta b1 lies far behind the line BC; seta c1 lies above and seta c2 – below trichobothrium C. Two modifications of this pattern were observed:
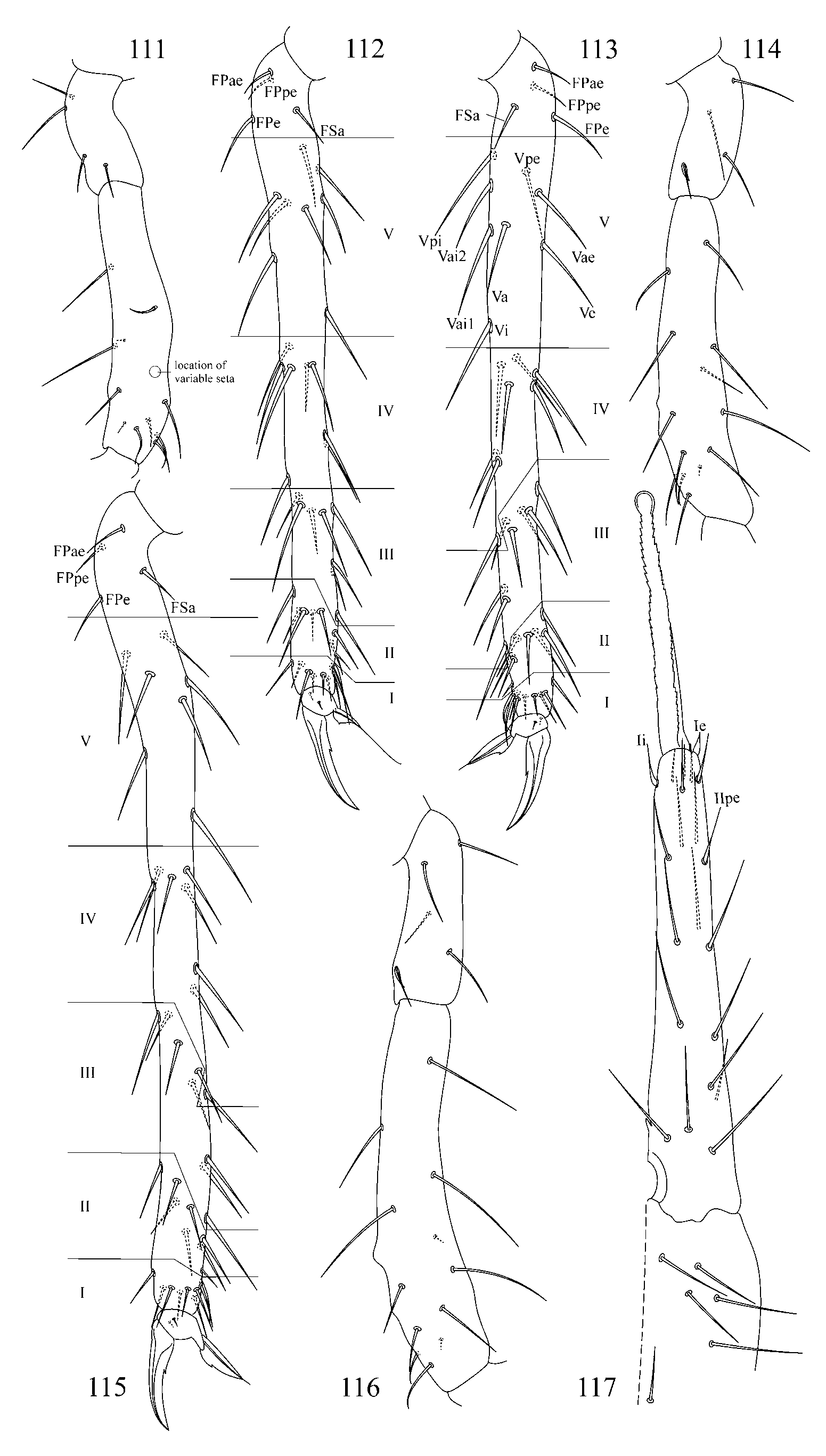
a) trichobothrium A is more distant from B than B from C. Such pattern is presently known for A. caecus ( Tullberg, 1871) (see in: Christiansen & Bellinger 1992: Fig. 142 View FIGURES 137 – 143 H; Fjellberg 2007: Fig. 111 View FIGURES 111 – 117 A; also my observation of Ukrainian material), A. ulehlovae Rusek, 1970 (see in: Rusek 1970: Figs 14 View FIGURES 2 – 18 , 28), and for A. karabiensis sp. nov. described here ( Fig. 4 View FIGURES 2 – 18 );
b) AB and BC are equidistant: shown for A. diversus ( Mills, 1934) (see in: Zeppelini 1999: Fig. 1), A. minor Park & Kang, 2007 , A. coreanus Park & Kang, 2007 (see in: Park & Kang 2007: Figs 1 b, 3b) and A. peculiaris sp. nov. described in this paper ( Fig. 36 View FIGURES 34 – 49 ).
1.2. Pattern of pygmaeus type ( Figs 65 View FIGURES 63 – 76 , 93, 120, 146): trichobothria ABC form a very obtuse angle, often up to linear mode (trichobothrium B is only slightly out of line AC or in line with AC). AB and BC are equidistant or AB may be somewhat shorter than BC. Additional characters: single p seta of p-row of Abd I is located below the level of trichobothrium B anteriorly to the trichobothrial complex (marked with arrow); seta b1 lies close to the line between trichobothria B and C; seta c1 of thichobothrial complex lies on the level or below the level of trichobothrium C. This pattern was observed in species from pygmaeus -group sensu lato (further divisions of species groups followed by Bretfeld 1999: 66):
pygmaeus -group sensu stricto: after original observation in A. pygmaeus ( Wankel, 1860) and A. cf. pygmaeus (specimens from Ukraine, Romania, Slovenia and the USA), A. terricola Gisin, 1958 (specimens from Ukraine), A. ruseki Nosek, 1975 (specimens from Ukrainian Carpathians), A. kristiani Vargovich, 2005 (see in: Vargovich 2005: Fig. 1, 1), A. aggtelekiensis Stach, 1945 and A. aggtelekiensis buekkensis Loksa, 1969 (specimens from Hungary and Slovakia) and 2 species described here ( tauricus and kaprusi ); after literature data in A. miravetensis Baquero, Herrando-Pérez & Jordana, 2005 (see Baquero et al. 2005: Fig. 3 View FIGURES 2 – 18 A) and A. styriacus Nosek & Neuherz, 1976 (see in: Nosek & Neuherz 1976: Fig. 1). Pattern is close to linear.
principalis -group: A. benitus ( Folsom, 1896) (see in: Christiansen & Bellinger 1992: Fig. 141 View FIGURES 137 – 143 E), two Crimean taxa described here ( pseudoprincipalis and principalis skelicus ) and two species from Caucasian caves. Following pattern has been observed: B somewhat out of AC and AB≤BC.
furcatus -group: A. bifidus Stach, 1945 (specimens from Ukraine and Hungary) and A. carpathicus Vargovich, 1999 (from Ukrainian Carpathians). Pattern is close to linear (B only slightly out of line AC), AB ≤ BC.
cochlaerifer -group: A. spinosus Rusek, 1967 (specimens from Slovakia and Ukraine), A. gisini Nosek, 1960 (see in: Nosek 1960: Fig. 6 View FIGURES 2 – 18 ). In this group the pattern is the farthest from linear and ABC angle is about 130–140º.
postumicus -group: A. nigripes Park & Kang, 2007 (see in: Park & Kang 2007: Fig. 2 View FIGURES 2 – 18 b). Pattern is almost linear, AB ≤ BC.
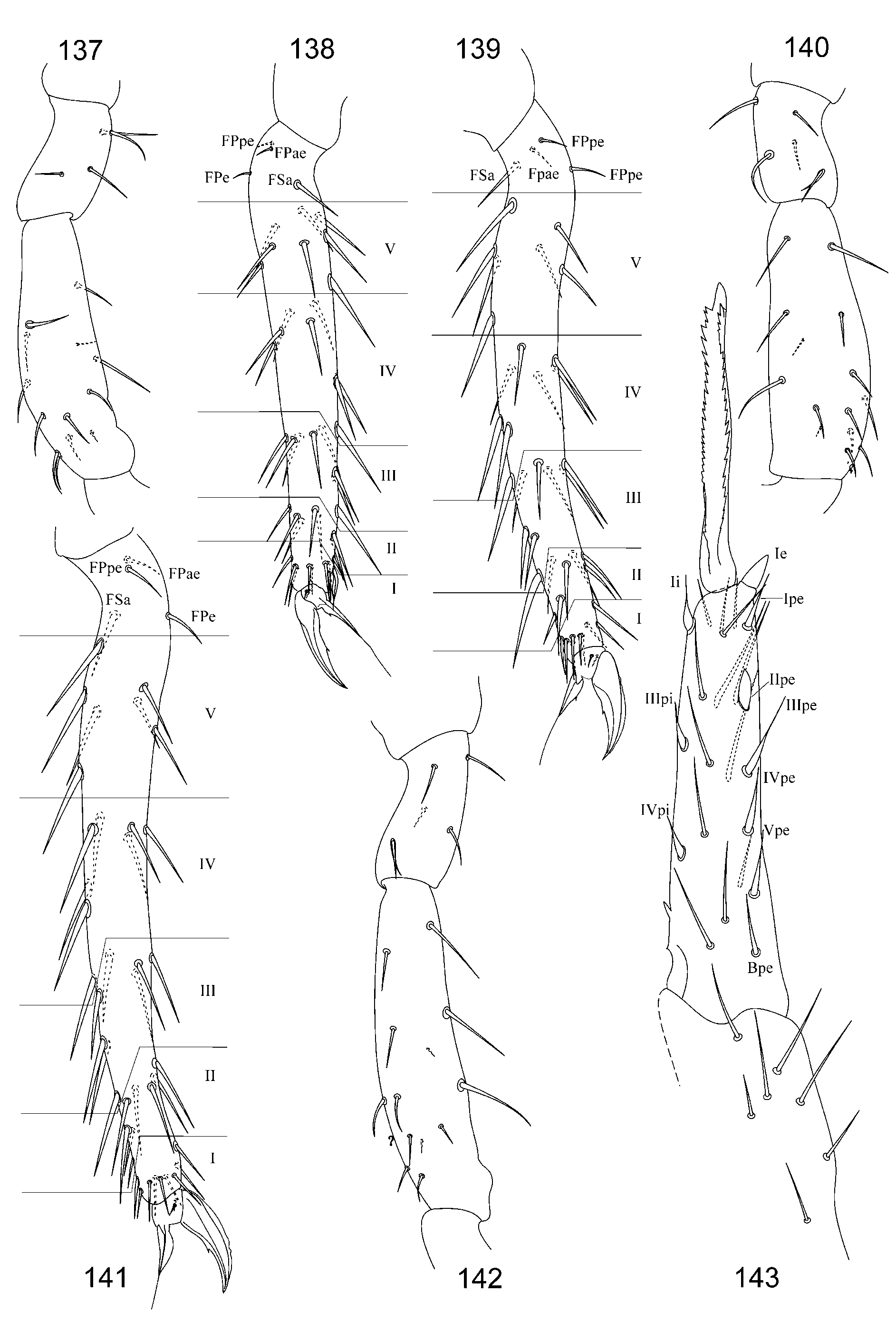
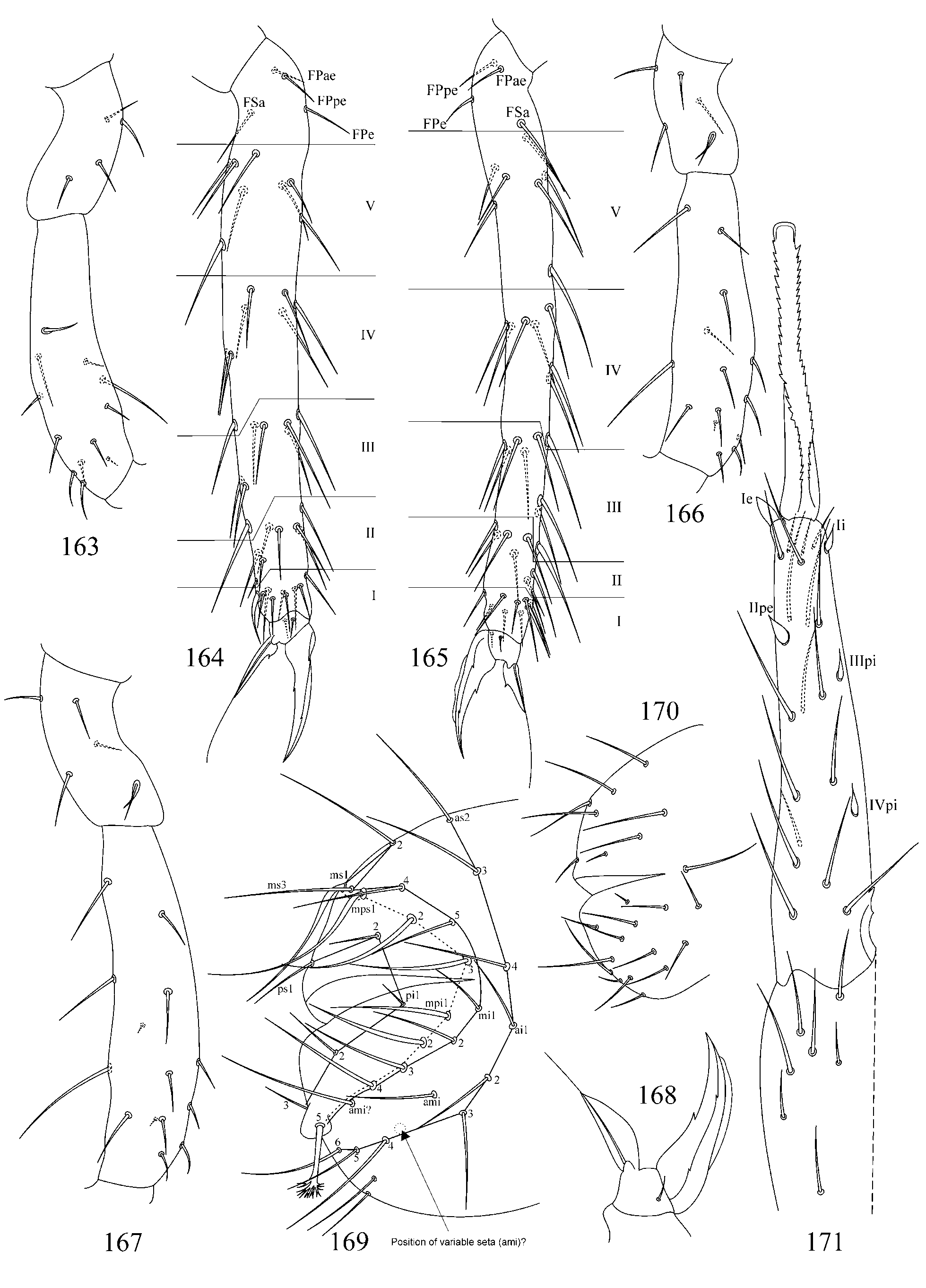
2. Tibiotarsal chaetotaxy of Symphypleona and particularly of Arrhopalites was studied by Nayrolles (1988) (on example of A. terricola ) and for this genus had been poorly known before. Since then several authors have described the chaetotaxy of tibiotarsi ( Baquero et al. 2005; Christiansen & Bellinger 1996 (only metatibiotarsi); Lin & Chen 1997; Nayrolles 1990a; Park & Kang 2007 (only metatibiotarsi); Vargovich 1999, 2005; Wu & Christiansen 1997). This literature data together with my own observation allow concluding that:
2.1. Secondary seta FS on fore and mid tibiotarsi is absent in the caecus -group (as in Figs 26, 27, 56, 57).
2.2. Species of the pygmaeus -group bear FS seta on all tibiotarsi (as in Figs 85, 86 View FIGURES 83 – 90 , 112, 113 View FIGURES 111 – 117 , 138, 139 View FIGURES 137 – 143 , 164, 165 View FIGURES 163 – 171 ), however, one exception is known in A. chiangdaoensis Nayrolles, 1990 (see in: Nayrolles 1990a: Table 1 View TABLE 1 ).
3. Anterior dens chaetotaxy has been justifiably used for determination of caecus and pygmaeus -group.
3.1. For the caecus -group it is very typical to have 5 setal rows on anterior dens, often with thickened or heavy spine-like (but in most species with simple) anterodistal seta (Figs 32, 33, 61). However, 4-rows pattern is also known in species which possess cuticular spines on anal valves and undoubtedly belong to the caecus - group: A. nivalis Yosii, 1966 a, A. baccettii Dallai, 1969 . Besides, intraspecific variations (4 or even 6 rows with normally 5 rows) also were observed (see variability in A. karabiensis sp. nov. and A. peculiaris sp. nov.).
3.2. Species of the pygmaeus -group possess 4 setal rows on anterior dens and anterodistal seta never as a spine. However, exception is also known: A. slovacicus Nosek, 1975 placed in pygmaeus -group s.str. ( Bretfeld 1999) possibly has 5 rows on anterior dens (see in: Nosek 1975: Fig. 6 View FIGURES 2 – 18 ). Moreover, the cases of variability also occur: the same aberrant specimen of A. kristiani which undoubtedly belongs to the pygmaeus - group, shows 4 setal rows on anterior surface of one dens and 5 rows on another dens ( Vargovich 2005). It seems that pygmaeus pattern (4 rows) of anterior dens chaetotaxy is an apomorphy in relation to caecus pattern (5 rows)––the result of loosing of 1 anterior seta.
4. Cuticular spines. Their presence on small abdomen is remarkable but often variable and not obligate character for caecus -group species (for example, as in A. ulehlovae , A. pukouensis , A. diversus , A. minor , A. coreanus and described here A. peculiaris ). None of species from pygmaeus -group has such spines.
5. Globular apex of mucro is the character of most species from the caecus -group, however, similar mucronal apex also was described in A. longicornis Cassagnau & Delamare, 1953 (see in: Cassagnau & Delamare 1953: Fig. 6 View FIGURES 2 – 18 G) from the pygmaeus -group.
Some other poorly known features may also serve as additional differential traits between considered groups: details of head chaetotaxy (clypeal area seems to be different in caecus- and pygmaeus -group: see descriptions below), etc.
In my opinion, despite some exceptions, the set of differences listed above is sufficient for splitting genus Arrhopalites into two separate genera. Actually, it is proposed to reanimate separate generic level for caecus and pygmaeus ( s.l.) groups on the base of newly defined and previously known diagnostic characters. However, as it was already mentioned, the name Coecarrhopalites introduced by Yosii as a subgenus and later as a genus ( Yosii 1954, 1967) is an objective synonym of Arrhopalites Börner, 1906 because the same type species was designated for both genera. Consequently, it is proposed to save generic name Arrhopalites only for caecus -group with type species Sminthurus caecus Tullberg, 1871 as it was originally designated by Börner, and to assign a new genus under the name Pygmarrhopalites for pygmaeus -group sensu lato with type species Dicyrtoma pygmaea Wankel, 1860 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Arrhopalites
| Vargovitsh, Robert S. 2009 |
A. kristiani
| Vargovich 2005 |
A. miravetensis Baquero, Herrando-Pérez & Jordana, 2005
| Baquero, Herrando-Perez & Jordana 2005 |
A. carpathicus
| Vargovich 1999 |
A. styriacus
| Nosek & Neuherz 1976 |
A. ruseki
| Nosek 1975 |
A. aggtelekiensis buekkensis
| Loksa 1969 |
A. terricola
| Gisin 1958 |
A. aggtelekiensis
| Stach 1945 |
A. bifidus
| Stach 1945 |
A. benitus (
| Folsom 1896 |
A. pygmaeus (
| Wankel 1860 |