Cylindrophis subocularis, Kieckbusch, Max, Mecke, Sven, Hartmann, Lukas & Ehrmantraut, Lisa, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4093.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:8C32F03F-E901-465D-B03D-7E6EEF288329 |
|
DOI |
https://doi.org/10.5281/zenodo.5662776 |
|
persistent identifier |
https://treatment.plazi.org/id/746A666D-D7EB-4FA7-A70B-8E42DEDFF3B8 |
|
taxon LSID |
lsid:zoobank.org:act:746A666D-D7EB-4FA7-A70B-8E42DEDFF3B8 |
|
treatment provided by |
Plazi |
|
scientific name |
Cylindrophis subocularis |
| status |
sp. nov. |
Cylindrophis subocularis sp. nov.
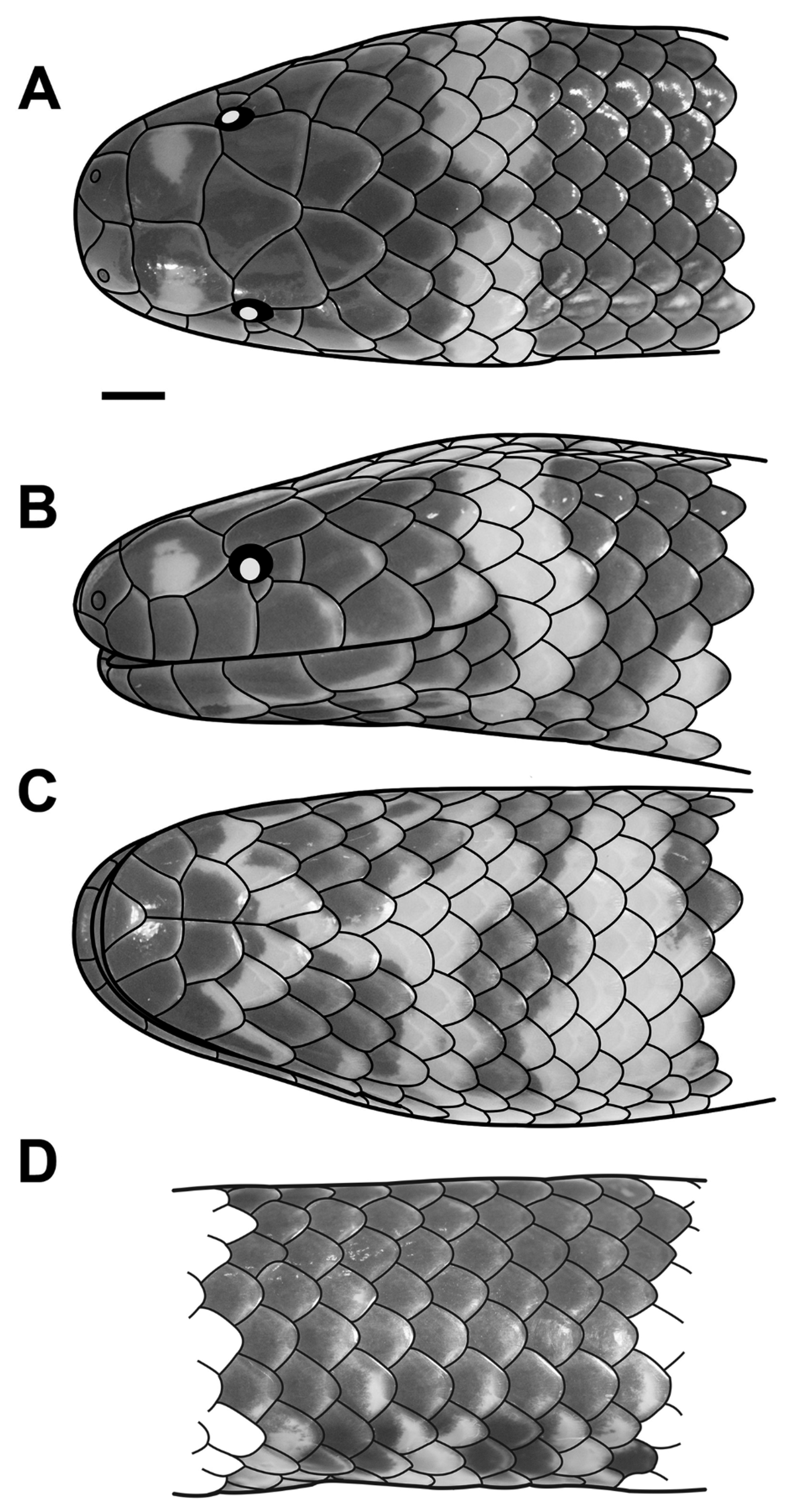
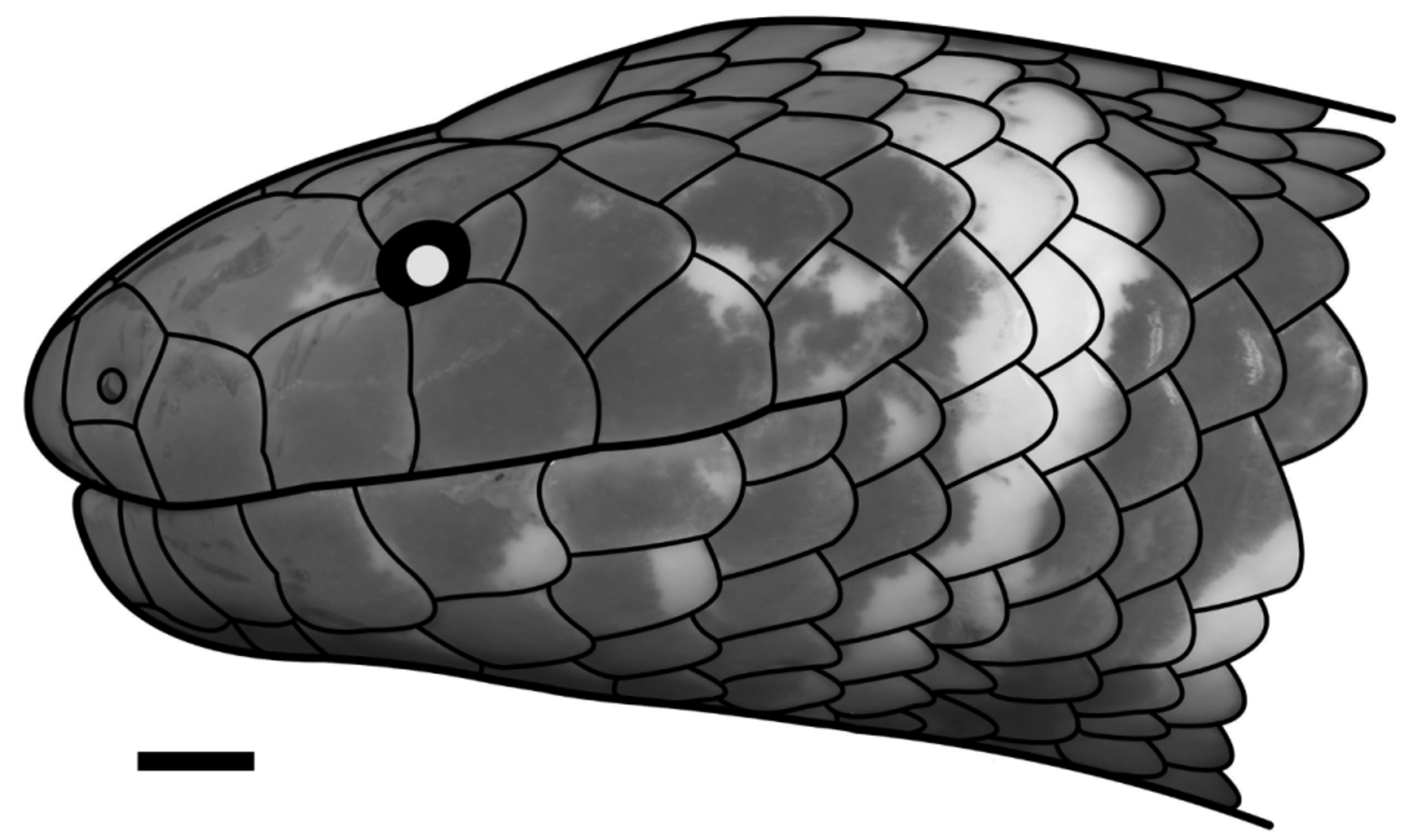
( Figs. 3−5 View FIGURE 3 View FIGURE 4 View FIGURE 5 ; Table 1 View TABLE 1 )
Holotype. RMNH.RENA 8785 ( Figs. 3−4 View FIGURE 3 View FIGURE 4 ; Table 1 View TABLE 1 ), an adult female, collected in Grabag, Purworejo Regency (formerly Koetoardjo), Central Java Province (Jawa Tengah), Java, Indonesia, by Felix Kopstein in February 1937. The original label for this specimen states “Grabag, Koetoardjo, Midden Java. + 10 m. ”
Paratypes. All RMNH.RENA specimens were collected by Kopstein at the type locality. RMNH.RENA 8958 ( Fig. 5 View FIGURE 5 A), a gravid female, was collected in October 1937; RMNH.RENA 8959 ( Fig. 5 View FIGURE 5 B), an adult female, was collected in November 1937; RMNH.RENA 11257 ( Fig. 5 View FIGURE 5 C), an adult male, was collected in August 1937; RMNH.RENA 11263 ( Fig. 5 View FIGURE 5 D), an adult male, was collected in August 1937; RMNH.RENA 47929 ( Fig. 5 View FIGURE 5 E), an adult male, was collected in November 1937. NMW 21559.1 ( Fig. 5 View FIGURE 5 F), an unsexed adult specimen from Java (no precise locality provided), was also collected by Kopstein, presumably during 1937, but the date is unknown.
Referred specimen. ZMB 53459, an unsexed adult with no further collection data.
Definition. A species of the genus Cylindrophis that can be readily distinguished from all congeners by the following combination of characters: (1) presence of a single subocular scale, positioned between 3rd and 4th or 4th 5. A Google search for the paper by Amarasinghe et al. (2015) by title leads to a downloadable pdf at the URL http://fds.lib.harvard.edu/fds/deliver/ 51488619/nsd_014410685_corrected.pdf. This URL features the term “corrected,” implying that an uncorrected version existed for download at least temporarily.
and 5th supralabial, contacting postocular and separating 4th or 5th supralabial from orbit ( Fig. 4 View FIGURE 4 B); (2) prefrontal in very narrow contact with or separated from orbit; (3) 19 smooth dorsal scale rows at midbody; (4) 6−7 supralabials; (5) 6−7 infralabials; (6) 190−196 ventrals; (7) 6−7 subcaudals; (8) 40−48 transverse light ventral blotches, and (9) light blotches on lateral surfaces of prefrontals ( Fig. 3 View FIGURE 3 A, 4A & B).
Comparisons. Cylindrophis subocularis sp. nov. can be easily distinguished from all congeners by the presence of a single subocular, positioned between the 3rd and 4th (rarely between the 4th and 5th)6 supralabial, contacting the postocular and separating the 4th (or 5th) supralabial from the orbit (e.g., Fig. 4 View FIGURE 4 B). In the following comparisons, ranges are followed by mean ± standard deviation and sample size (n), with the measures and counts for C. subocularis provided in parentheses. Whenever range and mean ± standard deviation are not provided, the respective character was invariable within a species.
Cylindrophis aruensis possesses 23 (19, n = 8) dorsal scale rows at midbody and 173–182 (190–196, 193.7 ± 2.0, n = 8) ventrals (Boulenger 1920; McDowell 1975; Amarasinghe et al. 2015). Cylindrophis boulengeri 6. While the general, relative position of the subocular is fixed, it may be bordered by the 4th and 5th supralabial, resulting from a vertical division of the 3rd upper labial.
possesses 197–204, 200.3 ± 3.5, n = 3 (190–196, 193.7 ± 2.0, n = 8) ventrals; and wavelike markings on supralabials, which may run onto prefrontals (uniformly dark supralabials and light blotches on prefrontals). Cylindrophis burmanus possesses 201−210, 208.3 ± 7.7, n = 6 (190–196, 193.7 ± 2.0, n = 8) ventrals. Cylindrophis engkariensis possesses 17, n = 1 (19, n = 8) dorsal scale rows at midbody; 230 7, n = 1 (190–196, 193.7 ± 2.0, n = 8) ventrals; rugose (smooth) dorsals on tail; a dorsal pattern of two paravertebral rows of spots (dorsal pattern of transverse, light, dorsolateral blotches); and uniformly colored prefrontals (light blotches on prefrontals). Cylindrophis isolepis possesses 21, n = 2 (19, n = 8) dorsal scale rows at midbody; and nasals separated by rostral (nasals in contact). Cylindrophis jodiae possesses 21, n = 77 (19, n = 8) dorsal scale rows at midbody; and wavelike markings on supralabials (uniformly dark supralabials). Cylindrophis lineatus possesses 21, n = 1 (19, n = 8) dorsal scale rows at midbody; 210 8, n = 1 (190–196, 193.7 ± 2.0, n = 8) ventrals; 9, n = 1 (6–7, 6.6 ± 0.5, n = 8) subcaudals; and a dorsal pattern of stripes (dorsal pattern of transverse, light, dorsolateral blotches). Cylindrophis maculatus does not possess light blotches on prefrontals (present); has a relatively longer snout, with SL/IOD = 1.03–1.25, 1.13 ± 0.06, n = 34 (0.94–1.03, 1.00 ± 0.03, n = 7); and a dorsal pattern of reddish-brown, large and round blotches (dorsal pattern of transverse9, light, dorsolateral blotches). Cylindrophis melanotus (including its synonyms Tortrix rufa var. celebica Schlegel, 1844 , T. rufa var. celebensis Gray, 1849 9, C. celebensis Smith, 1927 , and C. heinrichi Ahl, 1933 ) possesses 230–268, 245.3 ± 10.5, n = 35 (190–196, 193.7 ± 2.0, n = 8) ventrals; and predominantly light-colored supralabials, including a characteristic dark bar running down the supralabials below eye (completely dark supralabials and light blotches on prefrontals). Cylindrophis opisthorhodus possesses 23, n = 6 (19, n = 8) dorsal scale rows at midbody; and has a light dorsum with dark speckles forming two paravertebral rows and occasionally a discontinuous vertebral line (dorsal pattern of transverse, light, dorsolateral blotches). Cylindrophis ruffus sensu lato (including its synonyms Anguis striatus Gmelin, 1789 , A. scytale Russell, 1801 , C. resplendens Wagler, 1828 , and C. mirzae ), and C. rufa var. javanica Gray, 1849 (inferred from the relevant descriptions, drawings, figures, or examination of type material) do not have a subocular scale (present). Javanese C. ruffus sensu lato have the prefrontal usually in broad contact with the orbit ( Fig. 6 View FIGURE 6 ; Table 1 View TABLE 1 ), with PrefO/ED = 0.28–0.60, 0.38 ± 0.08, n = 51 (prefrontal in narrow contact with or separated from the orbit [ Fig. 4 View FIGURE 4 B]; with PrefO/ ED = 0.0–0.27, 0.11 ± 0.11, n = 8); results of Mann-Whitney U-test: Z = 0.29, p <0.001***. Cylindrophis yamdena possesses 21 (19, n = 8) dorsal scale rows at midbody, and a pale light dorsum without any pattern (Smith & Sidik 1998) (dorsal pattern of transverse, light, dorsolateral blotches).
Description of the holotype: metrics (in mm) and pholidosis. An adult female; SVL 385; tail very short, TL 10 (2.6 % of SVL); head not distinct from body; body cylindrical, body diameter 12.0 (3.1 % of SVL); head rounded in dorsal view; HL 11.9 (3.1 % of SVL); HW 8.7 (73.1 % of HL); snout rounded in dorsal and lateral view; SL 5.1 (42.8 % of HL); SW 3.4 (66.7 % of SL); ED 1.3 (10.9 % of HL); pupil round; IOD 5.0 (42.0 % of HL); NOD 3.7 (31.1 % of HL); PrefO/ED 0.04; internarial distance 2.5; pelvic spurs not visible externally but hidden in pouches situated laterally of cloacal plate, covered by scales; 21/19/17 dorsal scale rows, scales smooth, apical pits absent; 196 ventrals; six subcaudals + one terminal spine; cloacal plate divided; rostral clearly visible from above, triangular, wider than high (rostral height 2.0, rostral width 2.2); two pentangular nasals, height 1.9, length 2.6; nasal suture sinistral in respect to prefrontal suture; naris positioned close to the suture of nasal with first supralabial; postnasal absent; loreal absent; prefrontal in contact with 2nd and 3rd supralabial; preocular absent; rectangular subocular scale present, length 1.0, height 0.9; one pentangular postocular (length 1.1, height 1.4); temporal formula 1 + 2, anterior temporal larger than each posterior temporal (anterior temporal length 2.5, height 2.6; upper posterior temporal length 2.6, height 2.1); 6|7 supralabials: on right side of head: 1st smallest, 3rd largest, 2nd, 4th, 5th, and 6th equal in size, 2nd and 3rd in contact with prefrontal, 3rd in contact with orbit; on the left side: 1st smallest, 3rd largest, 4th, 5th, and 6th equal in size, 2nd, 3rd and 7th equal in size, 2nd, 3rd, and 4th in contact with prefrontal, 4th in contact with orbit; six infralabials, 3rd in contact with first pair of chin shields; first pair of infralabials in contact, preventing contact of mental with first pair of chin shields; mental triangular, wider than high, width 2.2, height 1.5; two pairs of chin shields, anterior chin shield length 2.1, width 2.0, posterior chin shield length 2.6, width 1.3; mental groove present, length 3.5; one hexagonal prefrontal, length 2.9, width 3.2; one pentangular supraocular, length 2.7, width 2.6; frontal rectangular, length 3.2, width 3.8; one pentagonal parietal, length 2.9, width 2.7.
7. Stuebing (1994) reported 234 ventrals for the holotype of C. engkariensis . A re-examination of the specimen by one of us ( HK) showed that there are only 230 ventrals present.
8. Blanford (1881) reported 215 ventrals for C. lineatus and Smith & Sidik (1998) provided a ventral range of 210−215. 9. Tortrix rufa var. celebensis Gray, 1849 is a nomen emendatum for T. rufa var. celebica Schlegel, 1844 and should currently be regarded a junior synonym of Cylindrophis melanotus Wagler, 1828 . It is also a junior secondary homonym of C. celebensis Smith, 1927 .
Description of the holotype: coloration and pattern in preservative (after 78 years in ethanol). Dorsal surface of head Sepia (279) with a Pale Buff (1) blotch on each prefrontal, extending from center of scale at about half scale’s width to lateral edge of scale; most upper head scales with lighter edges; supralabials Sepia (279); ventral surface of head Sepia (279) with lighter edges of scales and a Pale Buff (1) ‘X’-shaped marking beginning at level of lower edges of 3rd infralabial, extending to throat ( Fig. 4 View FIGURE 4 C); neck with a two scale broad Pale Buff (1) collar, interrupted medially in vertebral region, located one dorsal scale behind parietals; dorsal surfaces of trunk and tail Burnt Umber (48); dorsal surface of trunk with paired, occasionally slightly alternating, transversely arranged Pale Buff (1) blotches, approximately one scale broad, well-developed anteriorly and posteriorly, very faint or absent at central part of trunk; dorsal surface of tail with a Pale Buff (1) band that continues to the ventral surface, demarcating a Raw Umber (48) tail tip; ventral surface of trunk Raw Umber (280), with 43 transverse, alternating ventrolateral Pale Buff (1) blotches (two ventral scales broad at midbody); cloacal region and ventral surface of tail Pale Buff (1), with a Raw Umber (280) tail tip (from 4th subcaudal to terminal caudal spine), and Raw Umber (280) blotches on scales covering the cloacal spurs.
Intraspecific variation. Our assessment of the variation is based on the holotype and six paratypes (three males, three females, one unsexed specimen; Figs. 3 View FIGURE 3 & 5 View FIGURE 5 ; Table 1 View TABLE 1 ), with measurements provided in mm and listed including range and mean ± standard deviation and specimen numbers (n) in parentheses: SVL 288–451 (361.1 ± 53.7, n = 7); TL 7–11 (9.6 ± 1.3, n = 7); 21/19/17 (n = 5), 20/19/18 (n = 1), and 20/19/17 (n = 1) dorsal scale rows; 190−196 (193.8 ± 2.2, n = 7) ventrals; 6–7 (6.7 ± 0.5, n = 7) subcaudals; six (n = 5), seven (n = 1) or 6|7 (n = 1) supralabials; six (n = 5), seven (n = 1), or 6|7 (n = 1) infralabials; 4th supralabial in contact with orbit in specimens with seven supralabials (n = 2); subocular present on both sides of head in all specimens (n = 7); subocular may be fused with postocular (n = 1); subocular in contact with postocular, orbit and 3rd and 4th supralabial (in the case of the presence of six supralabials) or 4th and 5th supralabial (in the case of the presence of seven supralabials); subocular size: length on right side of head 0.6–1.6 (1.0 ± 0.3, n = 6) and 0.8–1.8 (1.1 ± 0.3, n = 7) on left side, height 0.6–1.7 (0.9 ± 0.4, n = 6) on right and 0.6–1.9 (1.0 ± 0.4, n = 7) on left side of head; 40–48 (43.1 ± 2.8, n = 7) alternating, light ventral blotches, two ventrals wide at midbody, three ventrals wide at midbody in a single specimen; light blotches on lateral surfaces of prefrontals might be fused into a bar running across the snout; light ‘X’-shaped marking on ventral surface of head might be dissolved into a reticulated pattern.
Etymology. The specific epithet subocularis is a compound adjective of sub (Latin: ‘under,’ ‘beneath’) and ocularis (Latin: ‘pertaining to the eye’), referring to the presence of a subocular scale in the new species.
Distribution and natural history. The new species is only known from Grabag on the south coast of Purworejo Regency, Central Java Province, Java, Indonesia ( Fig. 7 View FIGURE 7 ). The type locality in the South Central Java basin area is enclosed by mountain ranges to the north, west, and east, which include active volcanoes (Darman & Sidi 2000).
During the geological history of Sundaland, Java was connected to the islands of Borneo and Sumatra (Voris 2000; Sathiamurthy & Voris 2006; Wilting et al. 2012), and according to Natus (2005) many elements of the Javanese terrestrial vertebrate fauna descended from Bornean and Sumatran lineages that migrated to Java during or even before the Pleistocene and Holocene. Natus (2005) also identified eight endemism centers for terrestrial vertebrates in Java (Natus 2005: Fig. 4.22), which can be divided into two major groups: the lowlands in the northwest (immediately adjacent to Sumatra) and the eastern parts of Java, and the highlands of the Neogene- Quaternary volcanic arc that stretches longitudinally through the centre of Java. The South Central Java basin, however, has long been isolated to the north by the central volcanic chain (based on the maps presented in Sathiamurthy & Voris 2006) that may have largely prevented immigration events to the south, leading to vicariant evolution. Although the range of Cylindrophis subocularis is probably not restricted to Grabag, it may indeed exhibit a relatively limited distribution in the South Central Java basin and therefore should be regarded as a regional endemic.
Based on the lifestyle of congeneric species, we assume that Cylindrophis subocularis is semifossorial and preys mainly on elongate vertebrates (e.g., fishes, caecilians, skinks, and snakes: Schmidt 1928; Taylor 1965; Pauwels et al. 2000; Kupfer et al. 2003; pers. obs.), which are subdued by constriction (Greene 1983). Both the limited distribution and the secretive semifossorial lifestyle of C. subocularis may explain its apparent rarity in museum collections.
One specimen of the new species (RMNH.RENA 8958) contains eggs covered by a thin membrane. An incision into the membrane of one of the largest eggs (length 26.8 mm, width 13.3 mm) revealed the presence of an embryo (approximately at developmental stage 26−27, following Zehr 1962). We believe that this observation confirms that Cylindrophis subocularis is a viviparous species (sensu Blackburn 1994), with viviparity being the reproductive strategy for most, if not all, Cylindrophis species (de Rooij 1917; Smith 1943; McDowell 1975; Blackburn 1985; Brischoux et al. 2011). We also found one specimen of the closely related C. ruffus from Java (NMW 21558.6) that contains fully developed embryos. No further information is available on the biology of C. subocularis .
Remarks. While we discovered six of the seven type specimens of Cylindrophis subocularis in the collection of the RMNH, all of which were collected by Felix Kopstein (1893−1939) and accompanied by precise collection locality data, a single specimen was found in the collection of the NMW. For this specimen (NMW 21559.1) the collection locality is limited to “Java,” but the specimen label lists Felix Kopstein as the collector of the specimen. Based on specimen labels in the RMNH, Kopstein collected Cylindrophis specimens at other localities in Java, such as at “Indramajoe” (Indramayu, on the north coast of Central Java). We have examined these, as well as 113 additional Javanese specimens, and all lack a subocular scale and have the prefrontal usually in broad contact with the orbit. We believe that NMW 21559.1 is part of the series Kopstein collected on the south coast of Central Java, but deposited mostly in Leiden, with the single specimen deposited in the Vienna collection10. We discovered an additional specimen of C. subocularis in the Berlin collection (ZMB 53459). In the absence of a listed collection locality and collector’s name, we chose not to include this specimen in our type series.
Two specimens (RMNH.RENA 47931–32, formerly RMNH.RENA 8785.80–81) from the same original jar (jar number 8785) as the holotype (RMNH.RENA 8785, formerly RMNH.RENA 8785.51) and supposedly also collected at Grabag, are not conspecific with Cylindrophis subocularis . In the original catalogue of the herpetological section of the RMNH, we found the following entry:
“ De fles [8785] bevat nu 3 ex, zij zijn bewerkt door E.M.J. Jaspars en door hem voorzien van de nrs. 51, 80, 81. Mogelijk zijn de nrs 80 en 81 door bewerker bij vergissing in deze fles ondergebracht en zijn zij afkomstig van Buitenzorg [Bogor], Java. ”
[The jar [8785] now contains three specimens; they were examined by E.M.J. Jaspars and labeled with the numbers 51, 80, 81. Potentially, the numbers 80 and 81 have been misplaced in the jar by the researcher and they may have originated in Buitenzorg [Bogor], Java.]
We agree with the catalogue entry that RMNH.RENA 47931–32 (formerly RMNH.RENA 8785.80–81) were most likely misplaced in the jar; these specimens strongly resemble Cylindrophis ruffus from Bogor (n = 9) in having no subocular and the prefrontal in broad contact with the orbit, PrefO/ED = 0.42 and 0.47 respectively (vs. subocular present and prefrontal in narrow contact with or separated from the orbit in C. subocularis, PrefO /ED = 0.0–0.27, 0.11 ± 0.11, n = 8). An additional specimen (RMNH.RENA 11255), with greatly damaged anterior head scalation, but lacking a subocular scale, was supposedly also collected at the type locality of C. subocularis . Due to the consistent presence of a subocular scale in the Grabag population, we have reasonable grounds to believe that RMNH.RENA 11255 is also not conspecific with the new species. We believe that RMNH.RENA 11255 was most likely also misplaced or erroneously labeled, as was the case with RMNH.RENA 47931–32.
TABLE 1. Data for the individual type specimens of Cylindrophis subocularis sp. nov., and a comparison of this species with C. ruffus sensu lato from Java (data of specimens with precise collection locality shown only). Metric characters are given in mm. Ranges are followed by mean ± standard deviation (indicated in parentheses). An ‘ X’ indicates a fusion between the subocular and the postocular.
| RMNH.RENA 8785 | RMNH.RENA RMNH.RENA 8958 8959 | RMNH.RENA 11257 | |
|---|---|---|---|
| Status | Holotype | Paratype Paratype | Paratype |
| Sex | F | F F | M |
| SVL | 385 | 394 326 | 451 |
| TL | 10 | 9 10 | 11 |
| Dorsals | 21/19/17 | 21/19/18 20/19/18 | 21/19/17 |
| Ventrals | 196 | 194 192 | 195 |
| Subcaudals | 6 | 7 7 | 7 |
| Supralabials | 6|7 | 6 6 | 6 |
| Infralabials | 6 | 6 7 | 6 |
| Ventral bands light | 43 | 40 48 | 43 |
| Ventral bands dark | 43 | 40 48 | 43 |
| Subocular scale length | 1.0|0.8 | 0.8|1.0 0.6|0.9 | 1.6|1.8 |
| Subocular scale height | 0.9|0.6 | 0.6|1.1 0.6|0.9 | 1.7|1.9 |
| PrefO/ED | 0.04 | 0 0.02 | 0.27 |
| TABLE 1. (continued). | |||
| RMNH.RENA 11263 | RMNH.RENA NMW 47929 21559 | C. ruffus sensu lato (n = 53) | |
| Status | Paratype | Paratype Paratype | |
| Sex | M | M unsexed | - |
| SVL | 331 | 353 288 | 148–737 (356.1±143.8) |
| TL | 7 | 10 10 | 4–19 (9±3.3) |
| Dorsals | 21/19/17 | 20/19/17 21/19/17 | 19–23/19−21/15–19 |
| Ventrals | 196 | 194 190 | 179–225 (194.5±8.9) |
| Subcaudals | 7 | 7 6 | 5–7 (5.9±0.7) |
| Supralabials | 6 | 7 6 | 6 |
| Infralabials | 6 | 6|7 6 | 6 |
| Ventral bands light | 40 | 43 45 | 33–59 (45.9±6.0) |
| Ventral bands dark | 40 | 43 44 | 32–59 (45.2±5.8) |
| Subocular scale length | X|1.3 | 1.0|1.1 1.1|1.1 | - |
| Subocular scale height | X|1.0 | 1.0|0.9 0.9|1.0 | - |
| PrefO/ED | 0.25 | 0.21 0 | 0.28–0.6 (0.38±0.08) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |