Latiremus, BO
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2005.00188.x |
|
persistent identifier |
https://treatment.plazi.org/id/B26C3D16-B71F-FFFA-BAD1-1359FE31FD5A |
|
treatment provided by |
Diego |
|
scientific name |
Latiremus |
| status |
|
VALIDITY OF LATIREMUS BO | I C, 1969
Chappuis (1954a) established the genus Delamarella in a preliminary note, presenting a detailed text description of the type species D. arenicola . In a later report, Chappuis (1954b) supplemented this description by providing additional drawings and a discussion of potential relationships; however, having been unable to place the new genus with confidence in any existing family, he preferred to consider it incertae sedis (see also Chappuis, 1954c). This uncertain taxonomic position remained unchanged when Petkovski (1957) and Cottarelli (1971) added two more mediterranean species to the genus, D. karamani Petkovski, 1957 from Croatia, and D. galateae Cottarelli, 1971 from Sardinia.
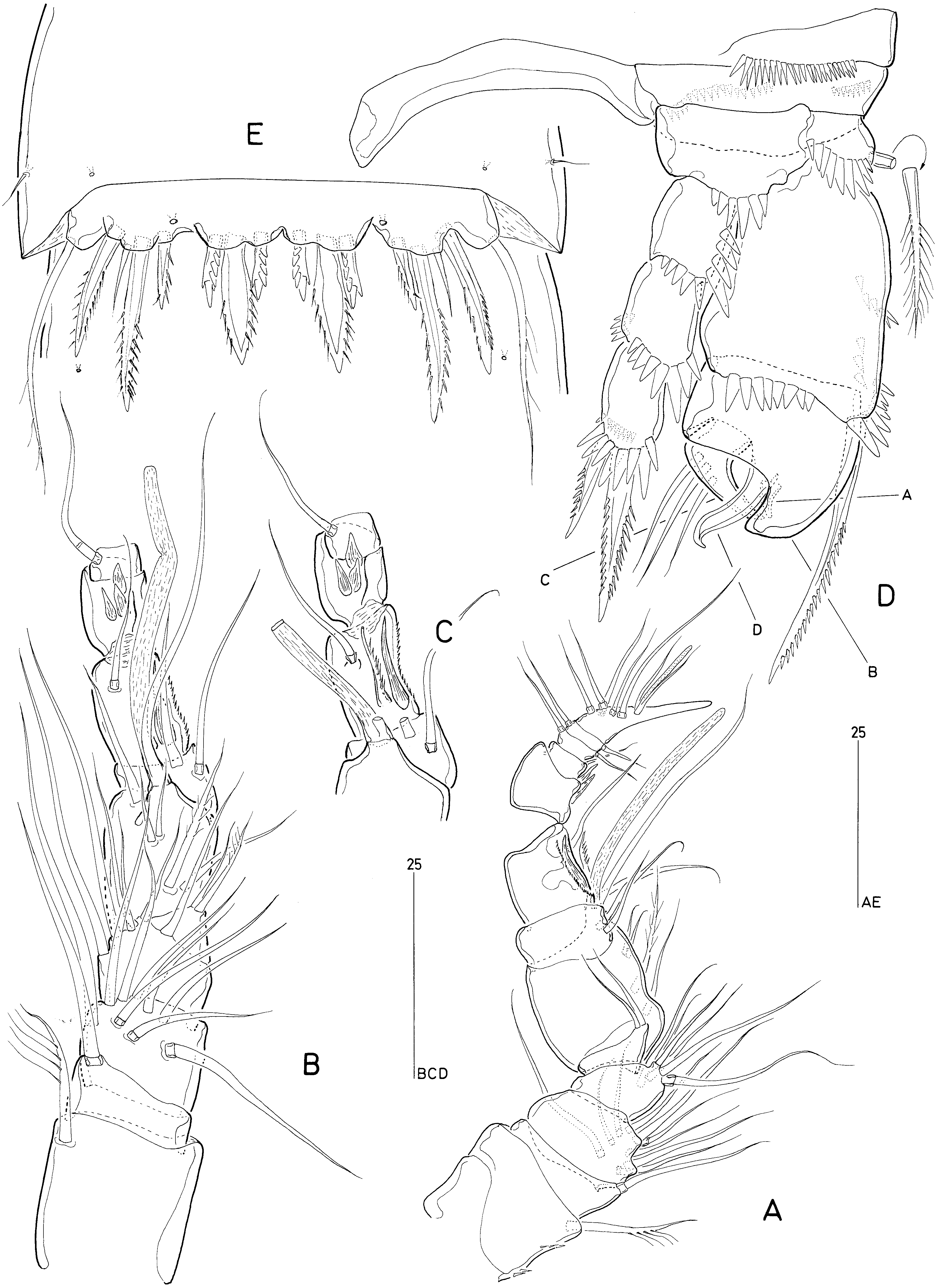
Bo | i c (1969) described a new genus and species, Latiremus eximius , from La Réunion, and considered it the type of a new family Latiremidae . As pointed out by himself (Bo | i c, 1978), he unfortunately overlooked the close affinity between Delamarella and Latiremus , a relationship first recognized by Itô (1974) and subsequently confirmed by various other authors ( Bodin, 1976a, b; Wells, 1976, 1978; Kunz, 1977). Most authorities considered the differences insufficient to maintain generic distinction and relegated Latiremus to a junior subjective synonym of the latter ( Apostolov & Marinov, 1988; Bodin, 1976a, b; Kunz, 1977, 1984; Bo | i c, 1978). This resulted in the family name being based on an invalid generic name, but as this course of action occurred after 1960, the validity of the family name and the designation of Delamarella as the type genus remained unaffected (ICZN: Art. 40). Wells (1976) preferred to treat them as distinct genera, an option also favoured by Huys & Kunz (1988) who redefined the generic boundaries within the Latiremidae . They reinstated Latiremus as a valid genus, moved Delamarella phyllosetosa Kunz, 1984 to a new genus Arbutifera , and restricted Delamarella to the three mediterranean species D. arenicola , D. karamani and D. galateae .
Huys & Kunz (1988) justified the separate generic status of Latiremus on the basis of the following characters: P1-bearing somite completely incorporated in cephalosome forming cephalothorax; genital and first abdominal somites completely free in ♀; seminal receptacles clearly separated; anal operculum with setulose frill but without spinules; caudal ramus setae II–III bearing subapical flagella; antennule ♀ 8-segmented; antennary basis and proximal endopod segment not fused; P1-exopod 3-segmented; P1 enp-1 with inner subdistal seta; P4 exopod ♂ with 3 setae and 1 strong spine; P5 without modified setae, exopodal lobe with 3 bipinnate spine plus seta in ♀ and 3 bipinnate spines in ♂. The following character states were used to diagnose Delamarella : P1-bearing somite partially incorporated in cephalosome; genital and first abdominal somites fused dorsally in ♀; seminal receptacles closely set; anal operculum with 10–15 spinules; caudal ramus setae II–III without subapical flagella; antennule ♀ 8- or 9-segmented; antennary basis and proximal endopod segment fused forming allobasis; P1-exopod 2-segmented; P1 enp-1 without inner subdistal seta; P4 exopod ♂ with 3 setae and at least 2 strong blunt processes; P5 middle seta of endopodal lobe with strips of serrate membrane, exopodal lobe with 3 bipinnate spine plus seta in both sexes.
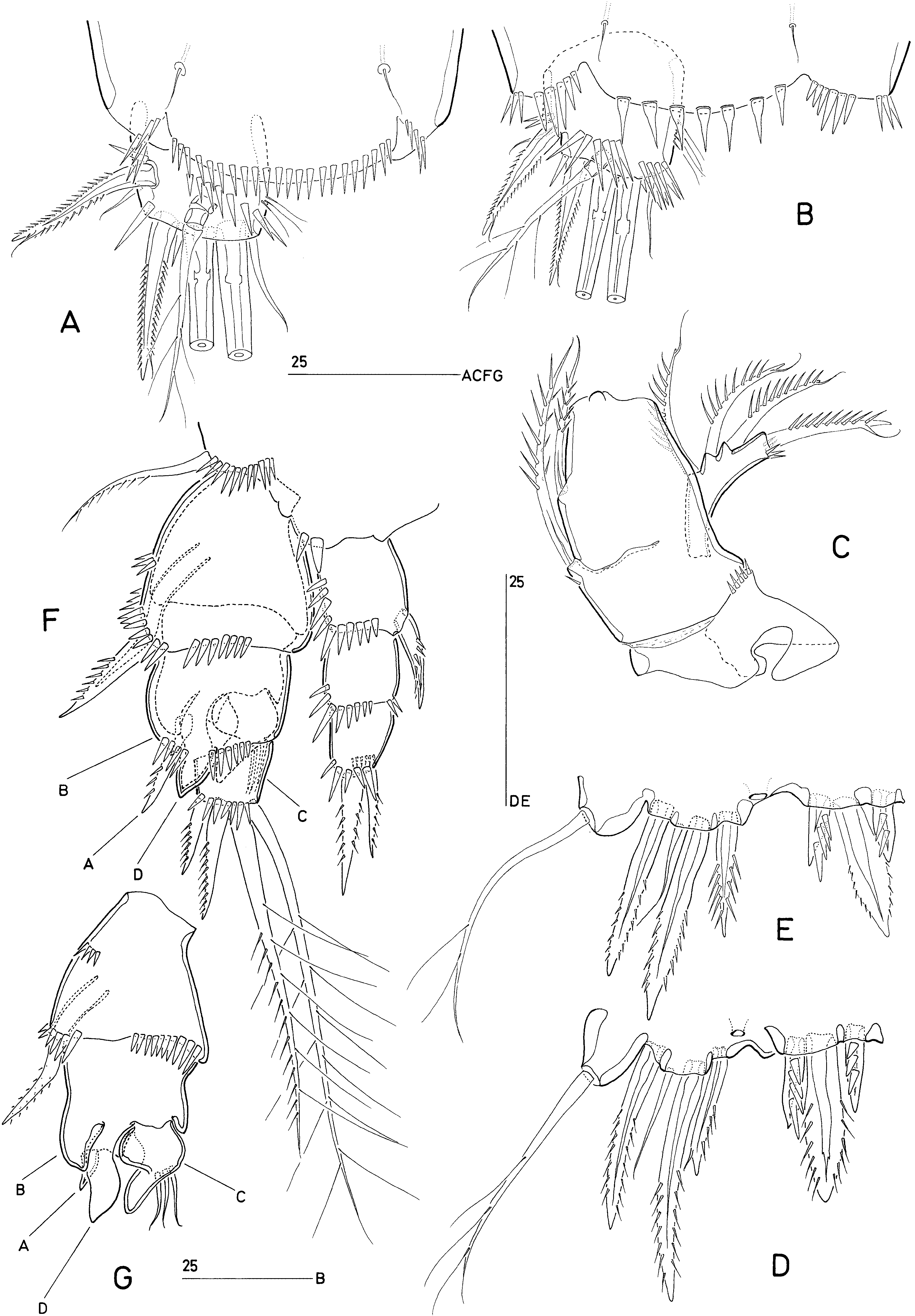
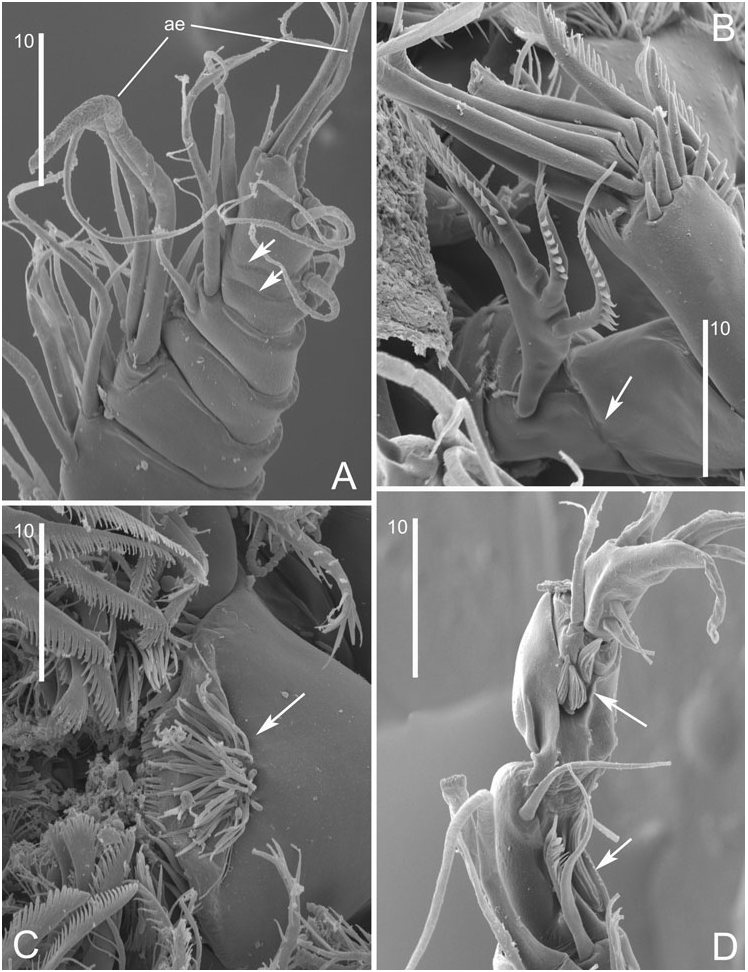
Our re-examination of D. galateae and description of D. obscura revealed that the characters used by Huys & Kunz (1988) to separate Delamarella and Latiremus are all essentially unsound and based on erroneous reports in the literature. Petkovski (1957) stated that the P1-bearing somite is only partly fused to the cephalosome in D. karamani and Cottarelli’s (1971) illustration of the male habitus of D. galateae appears to substantiate this. It now appears that both authors have wrongly interpreted the extensive intersomitic membrane separating the cephalothorax from the P2- bearing somite. It is conceivable that Kunz (1984) made the same observational error in his description of Arbutifera phyllosetosa and that consequently all latiremids possess a genuine cephalothorax. Similarly, Huys & Kunz (1988) extrapolated Petkovski’s (1957) observation of a dorsally fused (but ventrally separated) genital double-somite in D. karamani to all Delamarella species. This is contradicted by our observations of D. galateae and D. obscura ; in these species the genital and first abdominal somites are completely separated as in Latiremus and Arbutifera . Generic distinction based on seminal receptacle position has also proven unreliable as the structures illustrated by Cottarelli (1971) in reality refer to the crescent-shaped genital apertures ( Fig. 11A View Figure 11 ) and this is probably also the case for Bo | i c ’s (1969: fig. 4a) figure of the female genital field in L. eximius .
The ornamentation of the anal operculum in D. obscura is intermediate between the finely serrate condition displayed by L. eximius and the more spinulose state found in D. arenicola and D. galateae , indicating that this character has no significance at generic level. Caudal ramus setae II and III have a subapical flagella in D. galateae and D. obscura ( Fig. 7A, B View Figure 7 ), suggesting this structure was not only overlooked in other Delamarella species , but is actually a diagnostic character for the family. The level of segmentation expressed in the distal part of the female antennule shows intraspecific variability in D. obscura (compare Figs 3E View Figure 3 , 8A View Figure 8 ). Huys & Kunz (1988) remarked that the 8-segmented conditions in L. eximius and D. arenicola may not be homologous; however, given the generally weakly defined boundaries of the apical segments, this claim requires confirmation before it can be attributed taxonomic significance. A similarly overemphasized character is the presence/absence of an antennary allobasis. All published descriptions of Delamarella species invariably state that the basis is fused to the proximal endopod segment, forming an allobasis. Our observations confirmed the presence of a transverse surface suture ( Fig. 8B View Figure 8 ) in both D. obscura and D. galateae which resembles the faint articulation drawn by Bo | i c (1969) in his description of L. eximius .
The 2-segmented P1 exopod reported by Chappuis (1954a, b), Petkovski (1957) and Cottarelli (1971) was considered by Huys & Kunz (1988) as one of six autapomorphies defining the genus Delamarella . We observed that in D. obscura and D. galateae the P1 exopod is in reality 3-segmented although the segment boundary between exp-2 and exp-3 is not always clearly discernible. Unlike the articulation between exp-1 and exp-2, the joint between exp-2 and exp-3 is telescopic in nature and does not display the usual condylar reinforcements. The middle and distal segments are connected by a membranous intersegmental zone (arrowed in Fig. 10B View Figure 10 ) which enables the latter to be partly withdrawn in the former ( Fig. 10B View Figure 10 ). When exp-3 is fully exposed, the telescopic boundary is hardly discernible, creating the false impression that the ramus is 2-segmented ( Fig. 10B View Figure 10 ). Although the inner seta on P1 enp- 1 in A. phyllosetosa and L. eximius has consistently been claimed to be absent in previous Delamarella descriptions, we have shown it to be present in at least D. galateae and D. obscura . As this element typically arises from the posterior surface of the segment, we suspect that it may have been overlooked in D. arenicola and D. karamani .
The most striking apomorphy of latiremids is displayed by the complex morphology of the male P4 exopod. The different processes and elements of the distal part of the ramus cannot readily be homologized with their equivalents in the adult female. Prior to the final moult the P4 is essentially the same in both sexes, except that the proximal and middle exopod segments are already expanded in the male ( Fig. 7F View Figure 7 ). Examination of a copepodid V ♂ intermoult of D. obscura ( Fig. 7F, G View Figure 7 ) provided new insights into the reorganization and allometric growth of the male exopod. The outer lobate process of exp-2 (B) is the homologue of the expanded distal outer margin (proximal to the outer spine) of the segment. The outer spine of exp-2 is strongly reduced, being represented by a small spiniform element (A) arising from the posterior surface of the segment. The large attenuated structure on exp-2 (D), opposing the claw-like distal segment, is not a modified setation element but homologous to the outer distal corner of the segment. Exp-3 becomes reshaped into a triangular, curved segment bearing three short setae which are conceivably the homologues of the two distal setae and the distal outer spine in the female. The hook-like extension of exp-3 may be derived from the proximal outer spine, which became incorporated, but no evidence can be found in support of this assumption. The inner spine on enp-1 is expressed as in the female, showing its loss in the adult male is secondary .
The reported difference in the male P4 exopodal ornamentation between Latiremus and Delamarella is almost completely attributable to observational errors. Chappuis’s (1954a, b) illustration of D. arenicola shows no setation element on the middle segment but three hook-like spines on the distal segment. Using D. obscura as a reference for comparison ( Fig. 6D View Figure 6 ), it is obvious that the inner spine corresponds to the rudimentary distal segment, the middle and outer spines to the attenuated outer distal corners of the middle segment, and that the small outer spine of the middle segment was overlooked. Similarly, Bo | i c (1969) illustrated the P4 of L. eximius with no outer spine on exp-2 and three setae plus one spine on exp-3; this atypical pattern results from conflating the reflexed small third segment (three setae) and the spiniform distal outer corner of the middle segment (spine). Petkovski (1957) again presented a different interpretation for D. karamani , showing a recurved inner spine on the middle segment (in reality this spine is the reduced distal segment) and two spinous processes (derived from exp-2) plus two setae (derived from exp-3) on the alleged distal segment.
Given the difficulty in observing the serrate flanges of the setae on the fifth legs it is premature to attribute any significance to the absence of this character in L. eximius . Finally, we suspect the absence of the smooth seta on the P5 exopodal lobe in female L. eximius (but not in the male) is based on an observational error and does not necessarily reflect phylogenetic distinctiveness. No such sexual dimorphism is found in any Delamarella species. The explanation for this lack lies in the progenetic development of the P5, resulting in the persistence of the sexually undifferentiated copepodid IV condition in the adults. Because this scenario (early offset) is probably applicable across the family there is little evidence to accept the alternative pattern displayed by L. eximius . In conclusion, as there are no conclusive grounds left to maintain Latiremus as a distinct genus it is formally synonymized with Delamarella and, consequently, its type species is transferred as D. eximia (Bo | i c, 1969) comb. nov. Based on published records the genus appears to assume a ponto-mediterranean distribution with one outlier in the Western Indian Ocean (Bo | i c, 1969); however, one of us (V.C.) recently discovered another morphologically close congener from the Philippines, suggesting that Delamarella is probably Tethyan in distribution. Most mediterranean species are found interstitially in beach sands influenced by freshwater, i.e. at or near the mouth of rivers and streams. This low salinity preference probably enabled the genus to colonize other habitats in the oligohaline Black Sea.
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |