Hyperoche martinezii ( Müller, 1864 ), Muller, 1864
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3905.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:A47AE95B-99CA-42F0-979F-1CAAD1C3B191 |
|
DOI |
https://doi.org/10.5281/zenodo.6114488 |
|
persistent identifier |
https://treatment.plazi.org/id/AE418800-FFD9-FF86-FF3F-FDB1606CFD7A |
|
treatment provided by |
Plazi |
|
scientific name |
Hyperoche martinezii ( Müller, 1864 ) |
| status |
|
Hyperoche martinezii ( Müller, 1864) View in CoL
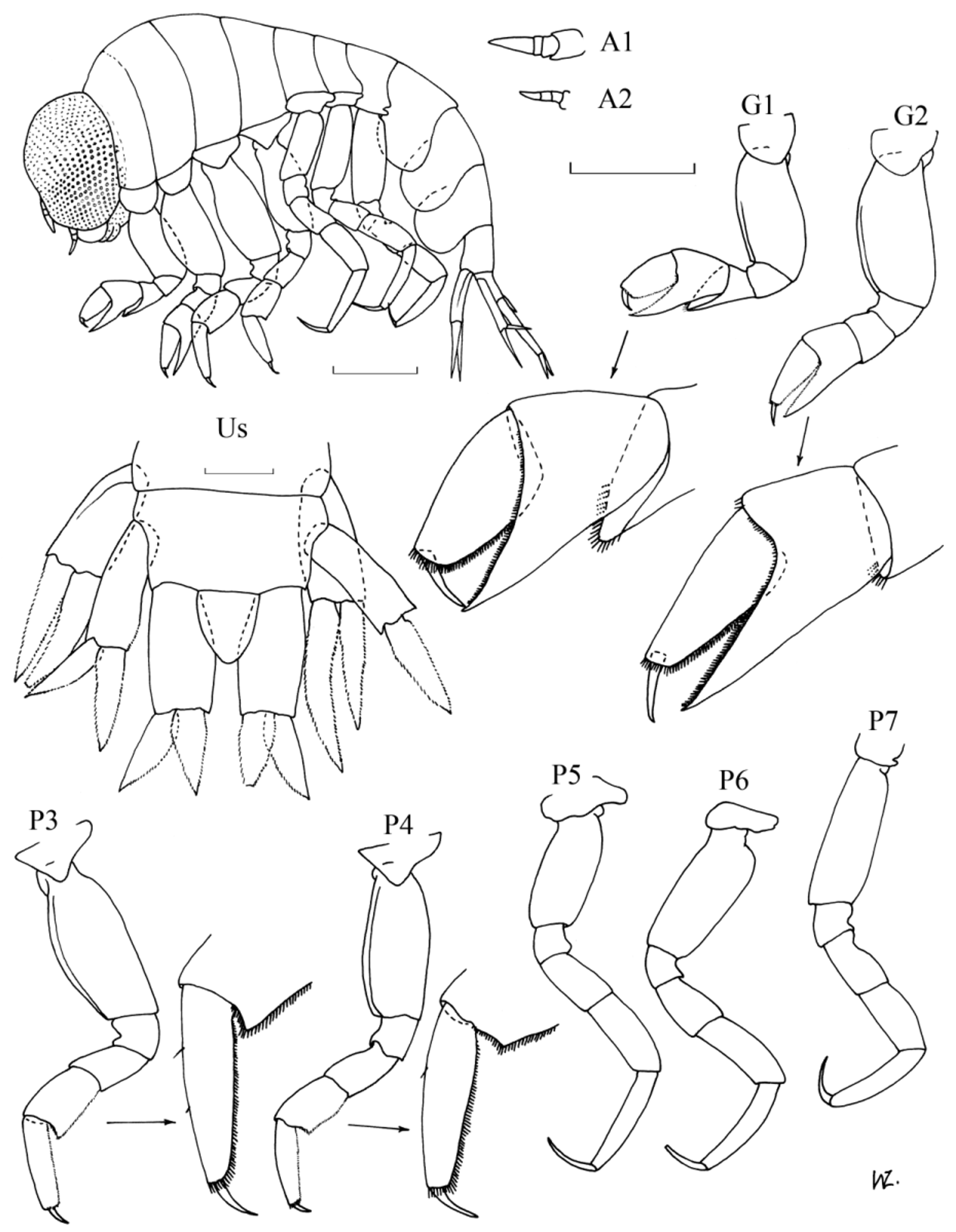
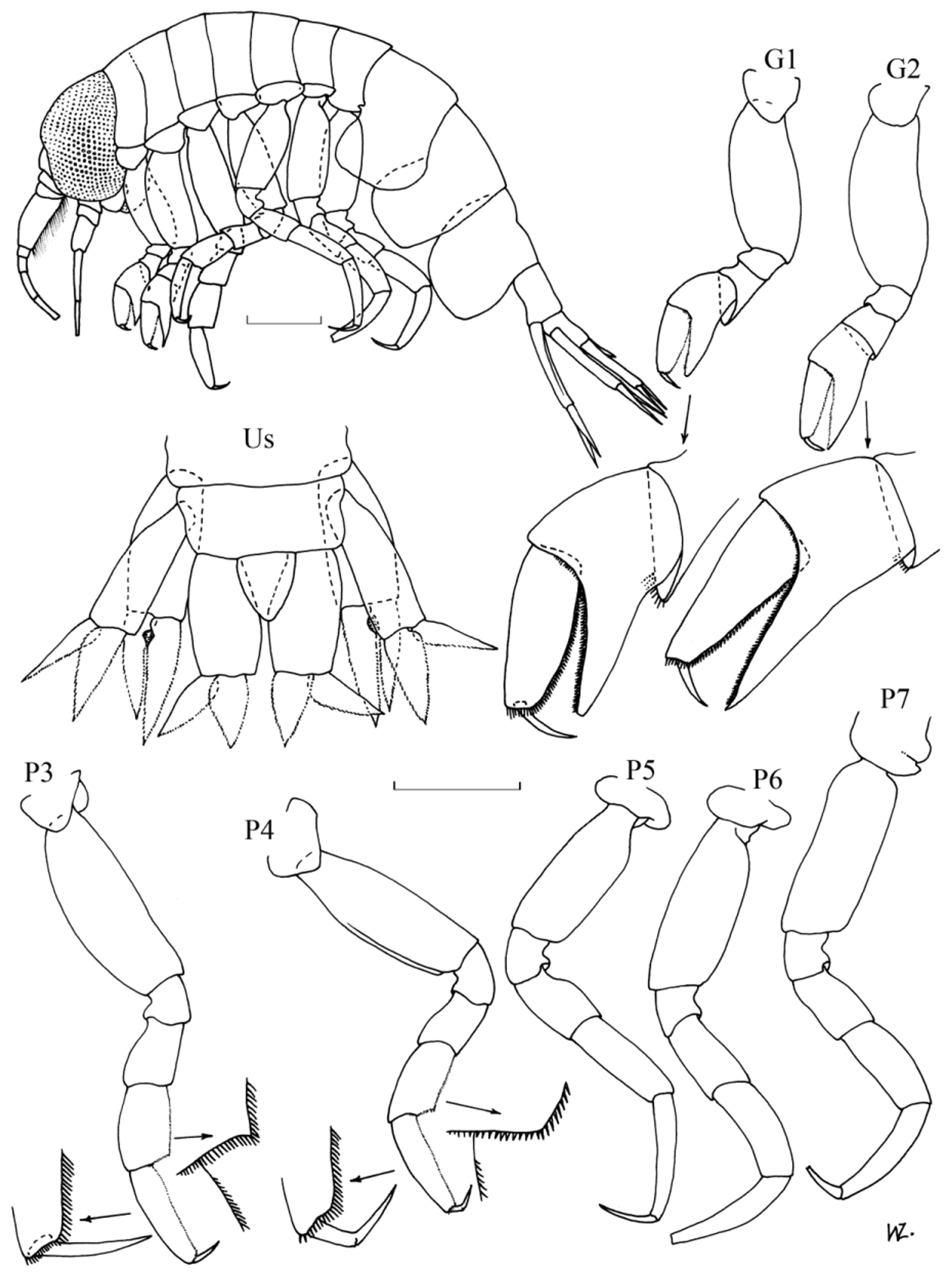
( Figs. 4–5 View FIGURE 4 View FIGURE 5 )
Hyperia Martinezii Müller, 1864: 51 View in CoL –52, text figs. 44–49.
Hyperoche Martinezi View in CoL — Bovallius 1887a: 19.
Hyperoche Martinezii View in CoL — Bovallius 1889: 86 (key), 107–111, text fig.; pl. 7, figs. 27–31.
Hyperoche martinezi — Steuer 1911: 674 –675 (key). Brusca 1981: 10 (list), 41, fig. 7h. Vinogradov et al. 1982: 282 (key), 286–287, fig. 145. Lin & Chen 1988: 324. Lin & Chen 1994: 115, 118 (table). Lin et al. 1995: 122 (list). Shih & Chen 1995: 84 (key), 86–87, fig. 51. Lin et al. 1996: 229 (table). Vinogradov 1999: 1146 (table). 1186 (incl. key), fig. 4.107. Lowry 2000: 325 (list). Brusca & Hendrickx 2005: 150 (list). Browne et al. 2007: 819 (table), fig. 4 (phylogenetic tree). Garcia-Madrigal 2007: 147, 191 (list). Gasca et al. 2010: 932. Gasca et al. 2012: 126 (table). Hurt et al. 2013: 31 (table), figs. 1–2 (phylogenetic trees).
Hyperoche martinezii View in CoL — Barnard 1930: 415 (key). Pereira 1962: 270 –272, figs. 1–10. Dick 1970: 36 (key), 57, fig. 6. Laval 1980: 18 & 23 (lists), 36–37. Lavaniegos & Ohman 1999: 494 (table). Escobar-Briones et al. 2002: 367 (list). Gasca 2009a: 88 (table).
? Hyperoche mediterranea View in CoL [mis-identification; all juveniles, most likely of H. martinezii View in CoL ]— Steuer 1911: 674 –677 (incl. key); pl. 1, figs. 1–5. Stephensen 1924: 79, fig. 33. Pirlot 1939: 37; pl. 2, figs. 5–6. Harbison et al. 1977: 467, 482 (table). Harbison et al. 1978: 239, 251 (table).
Type material. Type material of Hyperia martinezii is most likely lost but the MNHN, Paris, has a male specimen (Am 1429) donated to the museum by Müller, which may represent type material. This may have been the specimen described and illustrated by Bovallius (1889). The type locality is the south-west Atlantic, off Brazil at “Dest erro”.
Diagnosis. Females: Sexually mature at about 4–5 mm. Antennae 1 relatively short, about half as long as head, about twice as long as A2. Head length equal to first two pereonites combined. Pereon globular, length about 1.6 x pleon. Gnathopod 1; basis slightly shorter than remaining articles combined, merus spoon-shaped, projecting under carpus, almost to base of propodus, with fringe of setae on distal margin; carpal process extends just past distal margin of propodus, anterior margin denticulate; posterior and distal margin of propodus also denticulate; dactylus slightly curved, length about 0.4 x propodus. Gnathopod 2 slightly longer and more slender than G1 but similar in structure except that the merus is not produced under the carpus and lacks the spoon-shaped process. Pereopoda all of similar length. Pereopod 3; basis length almost 4 x merus; carpus with postero-distal corner produced in slight tooth with denticulate margins, length 1.4 x merus and 0.7 x propodus; posterior margin of propodus denticulate; dactylus length about 0.3 x propodus. Pereopod 4 similar in structure to P3 except the basis is relatively shorter, the carpus is relatively longer and the postero-distal corner of the merus is not as produced. Pereopods 5–7 are similar in size and structure. Pereopod 5; basis length almost twice merus; carpus length about 1.3 x merus, about 0.8 x propodus; dactylus length half propodus. Pereopods 6 & 7; like P5 but basis relatively longer, and P7 with coxa fused with pereonite. Epimeral plates with postero-distal corner rounded. Uropod 1; peduncle reaching just past middle of peduncle of U2 and just past base of peduncle of U3; inner ramus slightly longer than outer, slightly longer than peduncle. Uropod 2; inner ramus slightly shorter than peduncle, slightly longer than outer ramus. Uropod 3; inner and outer ramus of similar length and width, about 0.7 x peduncle. Telson triangular, marginally longer than wide, slightly longer than half of peduncle of U3.
Males: Sexually mature at about 5–6 mm. Antennae almost as long as entire animal. Pereon and pleon slender, of similar length. Appendages generally more slender than in females, especially the gnathopoda, otherwise very similar in structure and relative lengths of articles, except for the following minor variations. Gnathopoda with merus not projected as far under the carpus. Pereopods 3 & 4 with postero-distal corner of the carpus less prominent. Epimeral plates relatively much longer and deeper. Uropod 1; peduncle extends to 0.7 x peduncle of U2, to about half peduncle of U3; inner ramus slightly shorter than peduncle; inner margins of both rami with characteristic proximal excavation. Uropod 3; rami relatively broader and shorter, length about 0.6 x peduncle.
Material examined. Mediterranean Sea: Specimens recorded by Stephensen (1924) as H. medusarum ; 4 juveniles ( ZMUC), east of Mallorca [39°27’N 05°26’E], Thor stn. 116, 26 June 1910; male, off western Algeria [36°13’N 01° 28’W], Thor stn. 223, surface, 5 September 1910; male, east of Gibraltar [36°33’N 04°25’W], Thor stn. 227, 99 m, 6 September 1910. S.W. Atlantic: Two juvenile males ( SAMA C7932), just north of Rio de Janeiro, Brazil [21°11.2’S 40°30’W], R/V Almirante Saldanha stn. 6447, surface, 22 August 1984. S.E. Atlantic: Male ( ZMUC) [23°26’S 03°56’E], Dana stn. 3980 IV, 100 mw, 17 February 1930. South China Sea: Three females & female ( ZMUC), north of Borneo [06°55’N 114°02’E], Dana stns. 3688 II & IV, 3500 & 1000 mw, 8 April 1929. Female & female ( ZMUC), adjacent Luzon [11°43’N 121°43’E], Dana stns. 3734 II & IV, 600 & 100 mw, 27 June 1929. East China Sea: Female & female, male ( ZMUC), north of Taiwan [25°11’N 122°35’E], Dana stn. 3722 IV & V, 100 & 50 mw, 29 May 1929. N.E. Pacific: Six males ( ZMUC), Gulf of California, near Isla Angel de le Guarda [29°20’N 133°15’W], H. Lemche, surface, 9 April 1959. Juvenile female ( USNM 1196346), off California [36°22’N 122°06’W], R/V Point Sur, 100–200 m, W.E. Browne. S.W. Pacific: Between New Caledonia and New Zealand (4 lots; ZMUC); male [24°46.5’S 170°18.5’E], Dana stn. 3610 II, 600 mw, 7 December 1928; male & female, male [25°54’S 172°36.9’E], Dana stns. 3621 II & 3622 III, 200 & 100 mw, 8 December 1928; male [34°24’S 178°42.5’E], Dana stn. 3630 II, 2000 mw, 17 December 1928. Female ( SAMA C4585), Tasman Sea, off Newcastle, New South Wales [32°47’S 152°40’E], CSIRO R/V Warreen, stn. 3/40, 200–0 m, 11 January 1940. North Indian: Female ( ZMUC), south of Sri Lanka [04°26’N 85°21’E], Dana stn. 3906 IV, 300 mw, 20 November 1929. Female ( ZMUC), south of India [05°28’N 80°08’E], Dana stn. 3910 II, 600 mw, 23 November 1929. Female ( ZMUC), near Sri Lanka [06°36’N 79°06’E], Dana stn. 3913 III, 300 mw, 1 December 1929. Male & two males ( ZMUC), south-west of India [03°14’N 75°21’E], Dana stns. 3915 II & III, 200 & 300 mw, 3 December 1929. South Indian: Male ( SAMA C7933), Ningaloo Reef, north of Exmouth, Western Australia, S. Wilson, 9 July 1998. Male ( ZMUC), near Bali [09°09’S 114°47’E], Dana stn. 3804 III, 300 mw, 30 August 1929. Female ( ZMUC CRU- 20288), tropical western part [00°07’S 63°56’E], Dana stn. 3919 V, 50 mw, 8 December 1929. Male ( ZMUC), north-east of Durban [28°18’S 33°49’E], Dana stn. 3965 II, 200 mw, 17 January 1930.
Remarks. This is a relatively small species reaching sexual maturity at about 4–6 mm. Females often have the pereon more inflated than in other species, so that the head is relatively smaller. Sometimes this character is exaggerated in more mature specimens. In having rounded epimeral plates it resembles H. picta , H. mediterranea and H. macrocephalus sp. nov. Hyperoche picta is readily distinguished by the hooded dactyls of the gnathopoda and H. macrocephalus sp. nov. has a relatively large head, distinctive gnathopoda and other minor charcters that distinguish it from its congeners, as detailed under that species. Thus, H. martinezii is most similar to H. mediterranea , but in that species the merus of gnathopod 2 is produced slightly under the carpus; the distal articles of the gnathopoda and pereopoda are often very hirsute and the peduncle of uropod 1 is relatively longer, almost reaching the limit of the peduncle of uropod 2.
Regarding the records of H. mediterranea by Steuer (1911), Stephensen (1924) and Pirlot (1939), all of juveniles, the limited figures provided by these authors seem to represent juveniles of H. martinezii rather than H. mediterranea . In particular the relatively broad merus with the distinctive postero-distal corner of pereopod 3, and the second gnathopoda with the merus not produced under the carpus, are specific characters of H. martinezii . I have also examined the Thor material ( Stephensen 1924) and, although poorly preserved, the specimens are more readily identified with H martinezii than H. mediterranea . Similarly, the records of Harbison et al. (1977, 1978), from the Caribbean Sea region, are considered a misidentification because they were based on Steuer (1911) and Stephensen (1924).
This species has been recorded as an associate of the following ctenophores; Beroe gilva ( Müller 1864, Lavaniegos & Ohman 1999), B. forskalii ( Steuer 1911) , B. cucumis ( Harbison et al. 1977, 1978), Leucothea multicornis & Ocyropsis maculata ( Harbison et al. 1978) , and B. forskalii & Bolina hydatina ( Laval 1980, Lavaniegos & Ohman 1999).
Distribution. This is a relatively uncommon species, with few records from the world’s oceans, mainly from near-surface waters in tropical regions. In the Atlantic it has only been found off Brazil and off South Africa. It seems to be more common in the Pacific with records from the China Sea region and from southern California to Mexico. The Dana also collected specimens from the south-western Pacific, between New Caledonia and New Zealand, as detailed above, representing a range extension for this species. The only previous record from the Indian Ocean is off South Africa ( Dick 1970). The Dana also collected specimens from this region and also from near Bali and India, the latter being new records for the North Indian Ocean. There are questionable records from the Mediterranean Sea by Laval (1980) and the records of the probable misidentifications of Steuer (1911) and Stephensen (1924), noted here, and its occurrence there requires confirmation.
It has not been recorded from the North Atlantic, except for the suspect records of Pirlot (1939), from near Newfoundland and the Bay of Biscay, and Harbison et al. (1977, 1978), from the Caribbean Sea, which is surprising considering the many historical expeditions conducted in the region. At least the latter is easily explained as a minor range extension from the type locality.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hyperoche martinezii ( Müller, 1864 )
| Zeidler, Wolfgang 2015 |
Hyperoche martinezii
| Gasca 2009: 88 |
| Escobar-Briones 2002: 367 |
| Lavaniegos 1999: 494 |
| Laval 1980: 18 |
| Dick 1970: 36 |
| Pereira 1962: 270 |
| Barnard 1930: 415 |
Hyperoche martinezi
| Hurt 2013: 31 |
| Gasca 2012: 126 |
| Gasca 2010: 932 |
| Browne 2007: 819 |
| Garcia-Madrigal 2007: 147 |
| Brusca 2005: 150 |
| Lowry 2000: 325 |
| Vinogradov 1999: 1146 |
| Lin 1996: 229 |
| Lin 1995: 122 |
| Shih 1995: 84 |
| Lin 1994: 115 |
| Lin 1988: 324 |
| Vinogradov 1982: 282 |
| Brusca 1981: 10 |
| Steuer 1911: 674 |
Hyperoche mediterranea
| Harbison 1978: 239 |
| Harbison 1977: 467 |
| Pirlot 1939: 37 |
| Stephensen 1924: 79 |
| Steuer 1911: 674 |
Hyperoche
| Bovallius 1889: 86 |
Hyperoche
| Bovallius 1887: 19 |
Hyperia Martinezii Müller, 1864 : 51
| Muller 1864: 51 |